Calculating Uncertainty is not an easy process. People find it challenging to estimate Uncertainty. This is why we have picked up the topic here so that you will get to know the procedure that will help you calculate it easily. All you need to do is focus on an eight-step process appropriately, and you will never find it difficult to calculate it quickly and adequately.
How to calculate Uncertainty?
- Specify the measurement process
Before getting into the calculation process, it is essential to have a plan. The planning is undoubtedly an excellent start to get the appropriate outcomes. First of all, you need to identify the measurement process. This will help you in uncertainty analysis and focus your kind attention on what matters the most.
How to specify the Measurement process?
Follow the below-mentioned instructions to specify the measurement process:
- Select the measurement function to evaluate
- Select the procedure or measurement method to be used
- Select the equipment to be used
- Select the desired range of measurement function
- Determine the test points to be evaluated.
- Identify and characterize Uncertainty resources.
Now that you have figured out the measurement processes to be evaluated, you need to identify the factors influencing Uncertainty in measurement results. However, this process is not an easy one, so be patient while working.
Finding Uncertainty sources
Finding uncertainty resources can be complicated and require a lot of time and effort. This stage is considered the most time-consuming process while evaluating the uncertainty measurement.
How to find Uncertainty sources
Follow the below-mentioned steps to find uncertainty sources
- Evaluate measurement process, calibration procedure, or test method
- Evaluate measurement equations
- Evaluate reference standards, reagents, and equipment
- Identify minimum required uncertainty resources
- Research for various information sources
- Consult an expert
- Quantify Uncertainty resources
Before moving the calculation of measurement uncertainty, you need first to determine each contributing factor’s magnitude. To attain this, you need to perform such data analysis and reduction.
How to Quantify Uncertainty?
Follow the below-mentioned steps to quantify the Uncertainty
- Collect data and information
- Select the correct data after appropriate evaluation
- Data analysis
- Quantify Uncertainty components.
- Characterize Uncertainty resources
Characterize each factor by a probability distribution and uncertainty type.
How to characterize Uncertainty sources?
Follow the procedure to characterize your uncertainty sources
- Categorize each uncertainty source: Type A or Type B
- Assign a probability distribution to each component
- Convert Uncertainties to standard deviations
After the probability distribution, identify the equation required to convert each uncertainty contributor to a standard deviation equivalent. This will help to reduce the uncertainty source to a 1-sigma level.
How to convert Uncertainty to standard deviations?
Follow the below-mentioned steps to convert uncertainty components to standard deviations
- Assign a probability distribution to each uncertainty sources
- Find the divisor for the selected probability distribution
- Divide each uncertainty source by respective divisor.
- Calculate Combined Uncertainty
After converting the uncertainty sources, it is time to calculate combined Uncertainty by the root sum of squares (RSS) method.
How to calculate the combined Uncertainty?
Follow the below-mentioned steps to calculate combined Uncertainty
- Square each uncertainty component’s value
- Add together all results obtained in the first step
- Calculate the square root of results obtained in step 2
- Calculate expanded Uncertainty
You have reached the phase where you are almost done with the uncertainty estimation.
How to calculate the expanded Uncertainty?
Follow the steps to calculate expanded Uncertainty
- Calculate combined Uncertainty
- Calculate Freedom’s effective degrees
- Select or Find a coverage factor (k)
- Multiply combined Uncertainty by a coverage factor
- Evaluate your Uncertainty budget
Now that you are done with the calculation of expanded Uncertainty, it is the best time to evaluate the uncertainty estimate for appropriateness. Ensure that your measurement uncertainty estimate appropriately represents the measurement process and is not under or over-estimated.



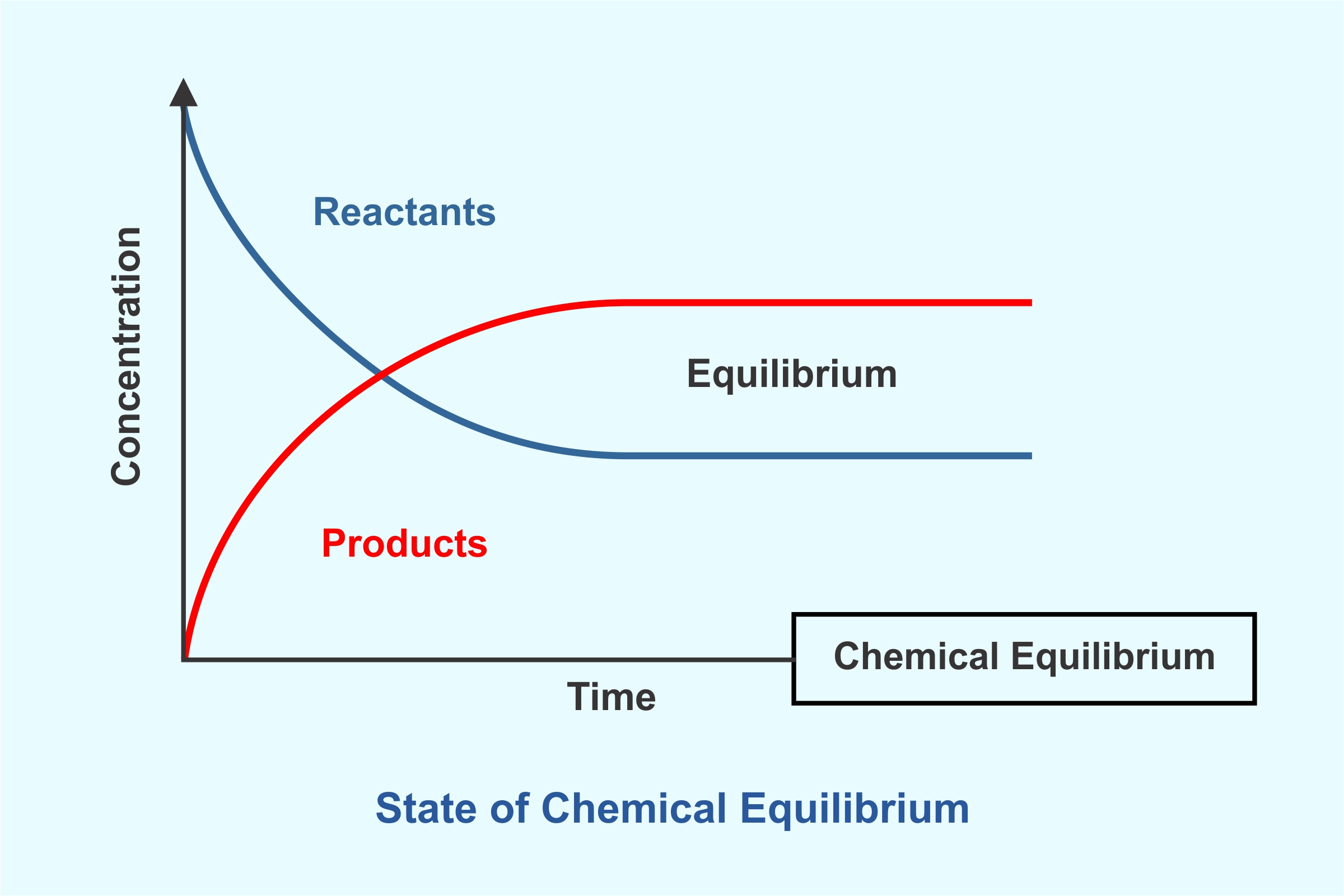
 State of Chemical Equilibrium
State of Chemical Equilibrium

