Enrich your knowledge with our informative blogs
What is equilibrium in chemistry?
Chemical equilibrium refers to the state of a system in which the reactant’s concentration and product’s concentration do not change with time. The system does not show any further changes in the properties.
The chemical equilibrium’s state is achieved by the system when the forward reaction rate is equal to the state’s reverse reaction. When there is no further change in reactants and product concentration due to equal rates of forward and reverse reactions, the system is said to be in the state of dynamic equilibrium.
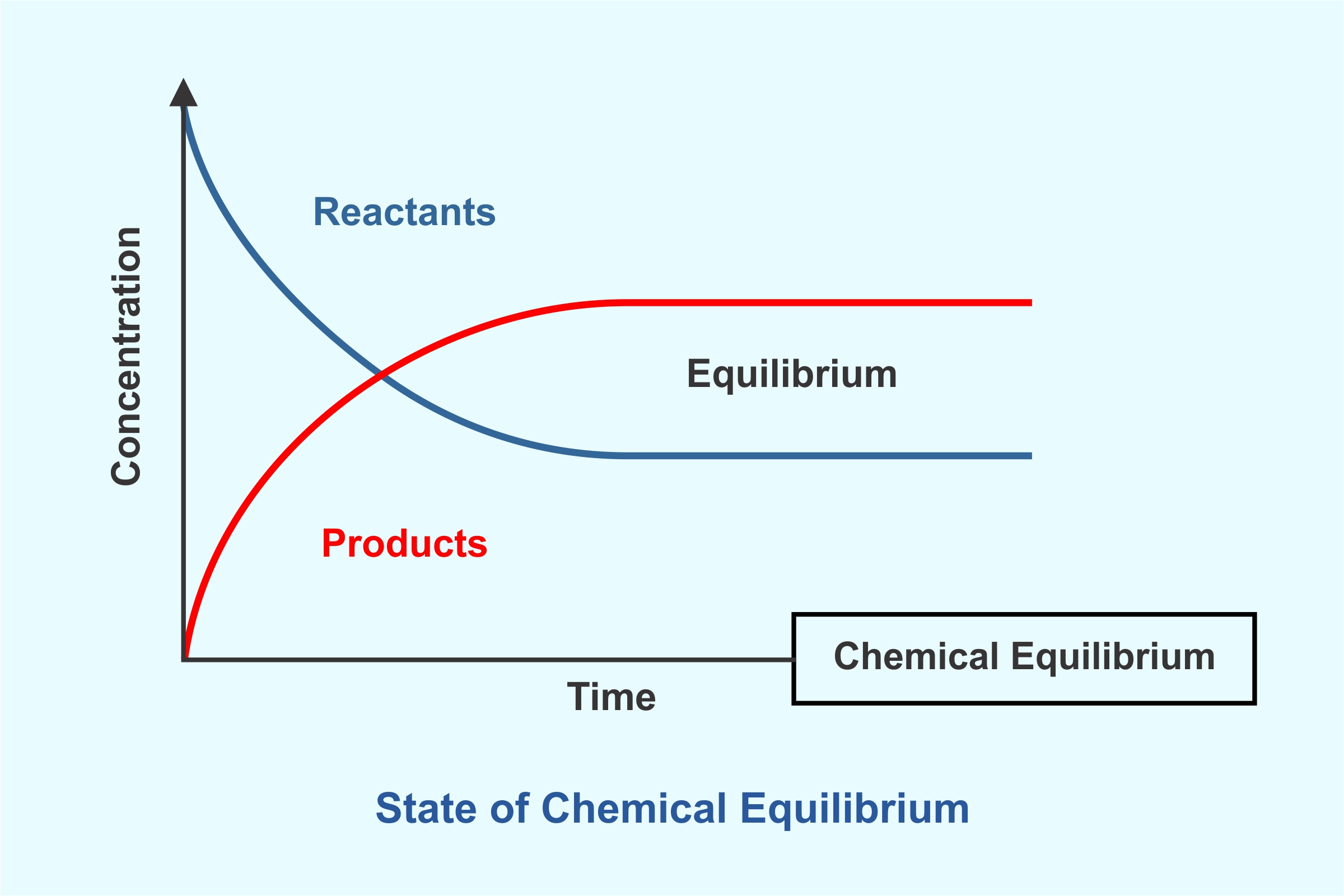
The graph with the time plotted on the x-axis and concentration on the y-axis indicates the chemical equilibrium is achieved once the concentration of both the products and reactants stops showing the change.
Types of Chemical Equilibrium!
Chemical equilibrium is of two types:
- Homogeneous Equilibrium
In Homogeneous equilibrium, the products and reactants of chemical equilibrium are all in the same phase. This type can further be divided into two types:
One is reactions, in which the number of molecules of a product is equal to the number of molecules of reactants. For instance:
N2 (g) + O2 (g) ⇌ 2HI (g)
Another is the reactions in which the product’s number of molecules is not equal to the total number of reactants number of molecules. For instance:
COCl2 (g) ⇌ CO (g) + Cl2 (g)
- Heterogeneous Equilibrium
In Heterogeneous chemical equilibrium, the products and reactants of chemical equilibrium are present in different phases. For example:
CO2 (g) + C (s) ⇌ 2CO (g)
Apart from these types, several factors affect Chemical Equilibrium. Let us get familiar with some essential factors affecting chemical equilibrium.
Factors affecting Chemical Equilibrium!
- Change in Concentration:
- When the concentration of the product or reactant is modified, there is a change in the mixture’s composition in chemical equilibrium.
- The products or reactants concentration added is relieved by the reaction which consumes the substance which is added.
- The products or reactants concentration removed is relieved by the reaction, which is in a direction that replenishes the substance which is removed.
- Change in Pressure
Change in pressure occurs due to a volume change. If there is a change in pressure, it can affect the gaseous reaction as the total number of gaseous products and reactants are now different. However, according to Le Chatelier’s principle, in heterogeneous chemical equilibrium, the change of pressure in both solids and liquids can be ignored as volume is independent of pressure.
- Change in temperature
Temperature’s effect on chemical equilibrium depends on the delta-H of the reaction and follows Le-Chatelier’s principle.
- In an endothermic reaction, the equilibrium constant increases with an increase in temperature.
- As the temperature increases, the equilibrium of exothermic reaction decreases.
Along with the equilibrium constant, the rate of reaction is also affected by the change in temperature.

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?