Iron and steel are the two most common materials that are often used in the manufacturing industry. They are generally used to make an extensive range of components and products.
While both the materials look somewhat similar, both are unique materials with their own respective qualities, characteristics, and properties.
Book Your 60-minutes Free Trial class NOW!
Let us understand the difference between both the materials but before that let us start with their definitions.
What is iron?
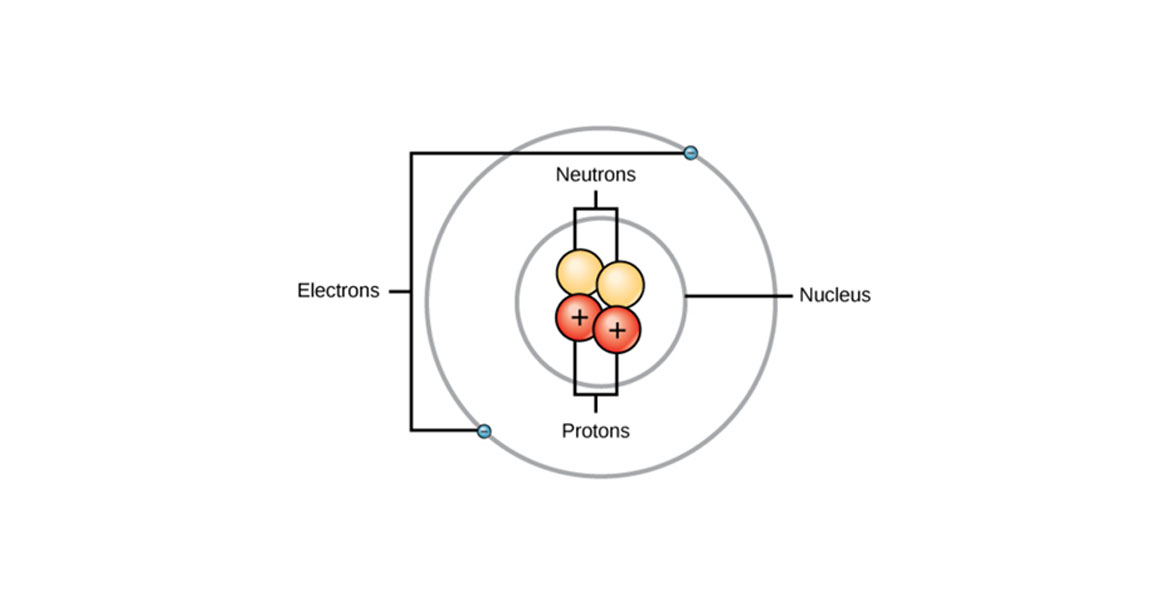
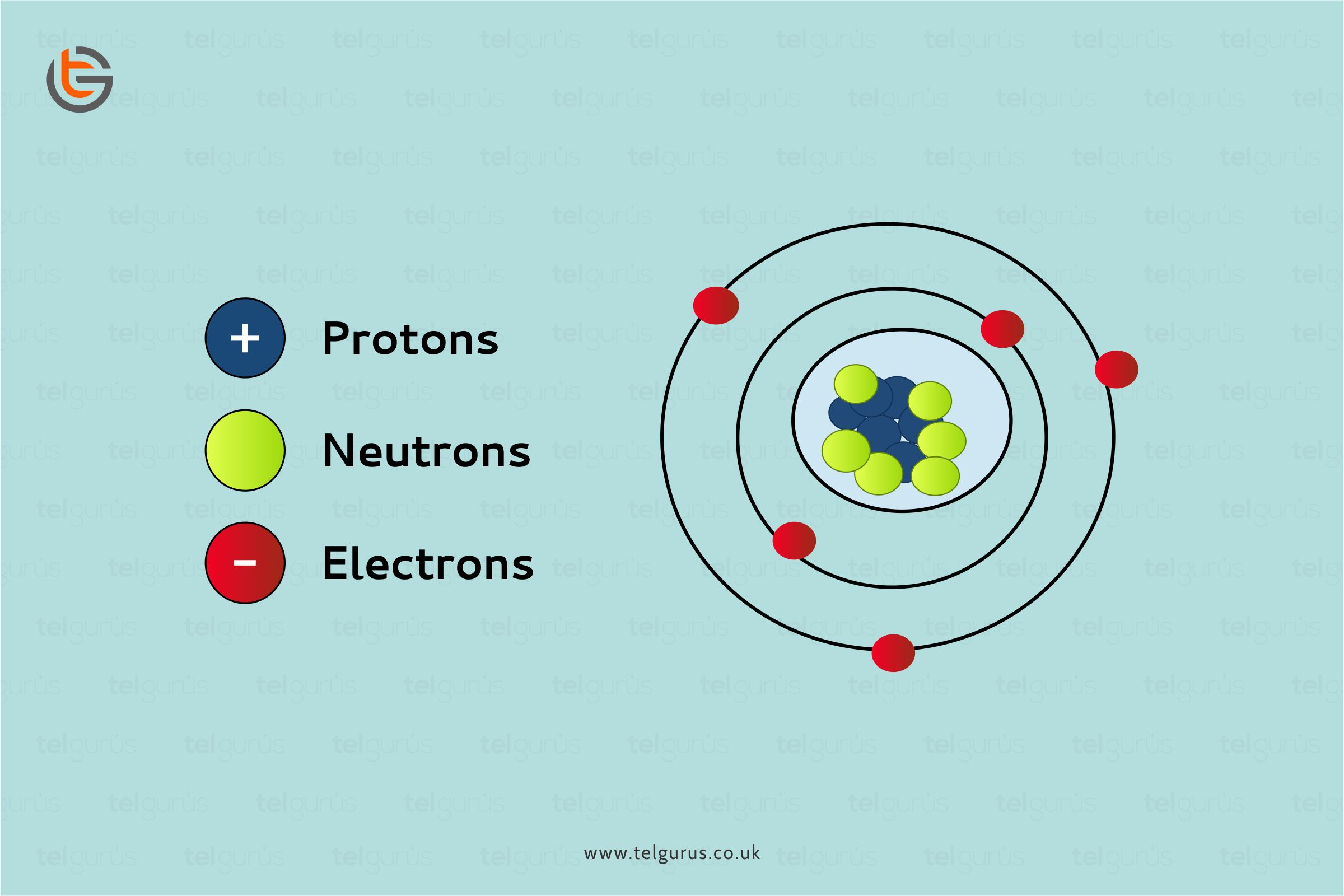
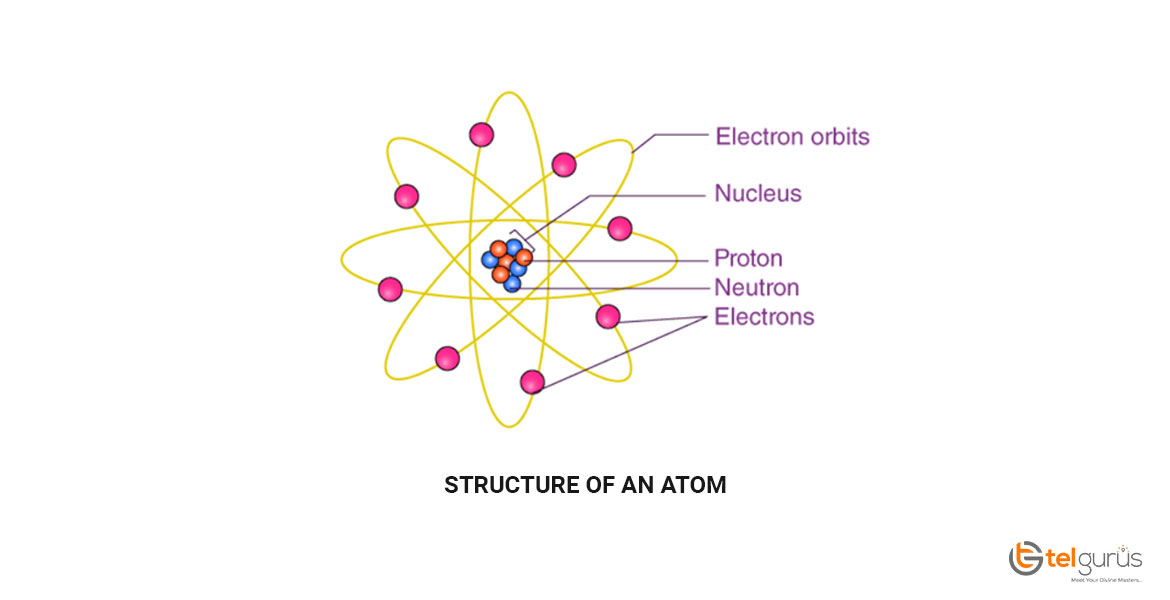
Iron is a ductile and lustrous metal having atomic number 26. Iron has a chrome-colored appearance reflecting a significant amount of light. It is also a ferromagnetic metal that means that it is magnetic and attracts other ferromagnetic metals.
Pure iron is generally silvery-white, cut through using a knife, and easy to work with. It can be hammered into the sheets and then drawn into wires. Despite so many surprising properties, iron still conducts electricity and heat like other metals and still can be magnetized.
What is steel?
Steel is basically a ferrous alloy comprising carbon and iron. Ample people assume that steel is metal but this is not necessarily true as it exhibits similar properties to metals but is technically classified as an alloy.
Steel has a lower carbon content than wrought or cast iron. It features several other elements to give it its properties.
Book Your 60-minutes Free Trial class NOW!
Differences between Iron and steel
Although both the materials begin with the same base compounds, post-production they transform into unique materials.
- Durability
Unlike iron, steel offers better durability and can withstand immense force, elements of weather, and heat.
- Corrosion
Corrosion is caused due to chemical oxidation and iron is susceptible to oxidation. Iron leads to corrosion and rust. While talking about steel it also gets affected by the water. Besides this, steel is basically a non-porous alloy that naturally resists corrosion.
- Versatility
Steel is considered an incredibly versatile material. It is well known for its flexibility and creativity and can be shaped, bent according to the requirement of any project and the best part is it can be reshaped without compromising on functionality.
While talking about iron, the versatility increases when mixed with some alloy like carbon.
Bottom Line!
The key difference between steel and iron is that their formal form is metal while the latter is an alloy. But both the metals are used in ample similar applications including the construction of railways, roads, buildings, utensils, and other appliances.
Book Your 60-minutes Free Trial class NOW!
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development