Enrich your knowledge with our informative blogs
How to calculate the number of neutrons, protons, and electrons in an atom of chlorine?

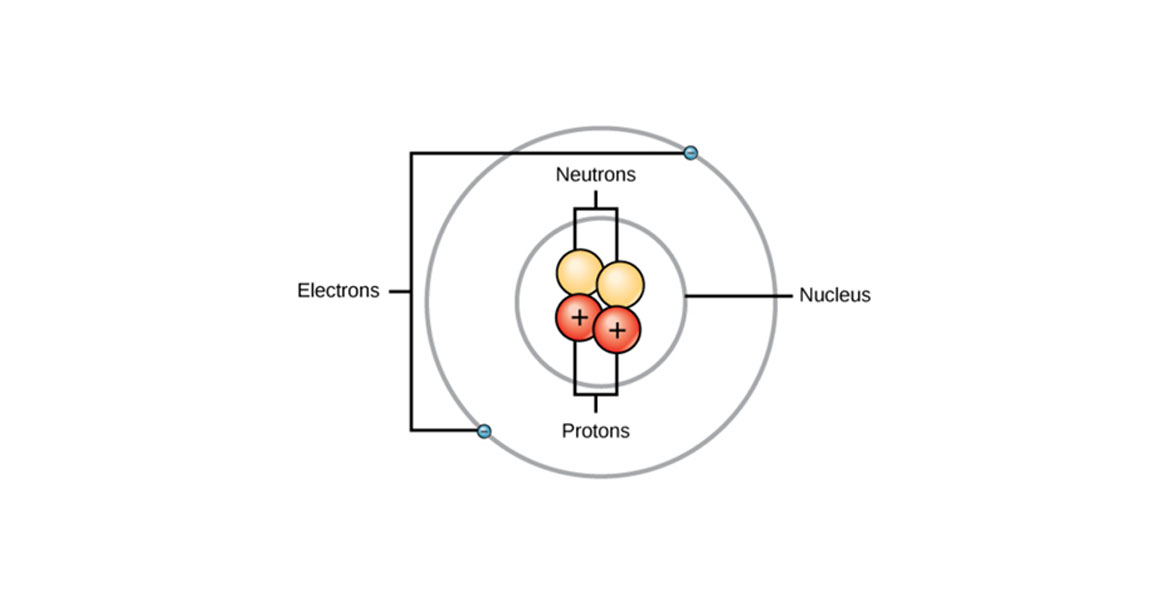
Atoms are generally made of extremely tiny particles known as neutrons, electrons, and protons. The neutrons and protons are present in the center of the atom that makes up the nucleus.
A proton contains a positive charge, whereas electrons are negatively charged. However, the charge on both electrons and protons is the same but opposite.
Before moving further, let us get acquainted with the definition of all three terms.
- Proton
Protons are positively charged subatomic particles that form the part of a nucleus of an atom. It also determines the atomic number of an element and weighs one amu.
- Neutron
Neutrons are the no charged subatomic particles forming the part of the nucleus of an atom. Its mass is equal to the mass of a proton and weighs one amu.
- Electron
Electrons are negatively charged particles surrounded by the nucleus. These weigh 0 amu.
Essential about protons, electrons, and neutrons
| Particle | Symbol | Charge | Mass |
| Electron | e– | -1 | 0.0005486 amu |
| Proton | p+ | +1 | 1.007276 amu |
| Neutron | n0 | 0 | 1.008665 amu |
As it is known that Protons carry +1 charge
Electrons carry a -1 charge
And the overall charge on an atom is 0
So, therefore, there must be an equal number of electrons and protons in an atom so that the charges cancel out.
Therefore if we take the case of Chlorine,
Calculating the number of neutrons, protons, and electrons in a Chlorine atom
The atomic number of Chlorine is 17
This indicates that each chlorine atom contains 17 protons and must also have 17 electrons.
An atom’s nucleus contains neutrons and protons. A nucleus is a primary contributor to the atom’s mass which means that the mass number can identify the number of neutrons and protons present in an atom.
Each neutron and proton contains a relative mass of 1 unit.
Therefore to the number of neutrons, we can subtract the number of protons from the atomic mass number.
Number of neutrons in Chlorine = Atomic mass number of Chlorine – Number of Protons
i.e., Number of neutrons = 35-17
Therefore, the number of neutrons in Chlorine= 18
Now that we know the number of protons in Chlorine = 17
Number of neutrons in Chlorine = 18
Let us check out the electrons
As it was said earlier, if Chlorine has 17 protons, it needs to have the same number of electrons.
Taking it that way,
Number of electrons = 17 + 1
Now, you must be thinking about why one is added here; well, it is because it has one negative charge that means the electron gain.
Therefore, the number of electrons = 18.
Conclusion
After calculating the protons, electrons, and neutrons, we have finally come to conclusive values of all. This includes
- Number of Protons = 17
- Number of Neutrons = 18
- & Number of electrons = 18
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?