Enrich your knowledge with our informative blogs
Explain the molecular structure of CH4
Methane is a gas consisting of one carbon atom for every four hydrogen atoms. Let us get acquainted with everything you need to know about the molecule structure of CH4.
What is Methane?
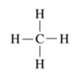
Methane is a gas comprising Carbon and hydrogen atoms with the chemical formula CH4.
It is found in small quantities in the atmosphere of the earth. It is defined as the simplest hydrocarbon comprising four hydrogen atoms and one carbon atom.
It is a powerful greenhouse gas that is flammable and is often used as fuel worldwide. It is primarily used to make light and heat.
Molecular Structure of Methane
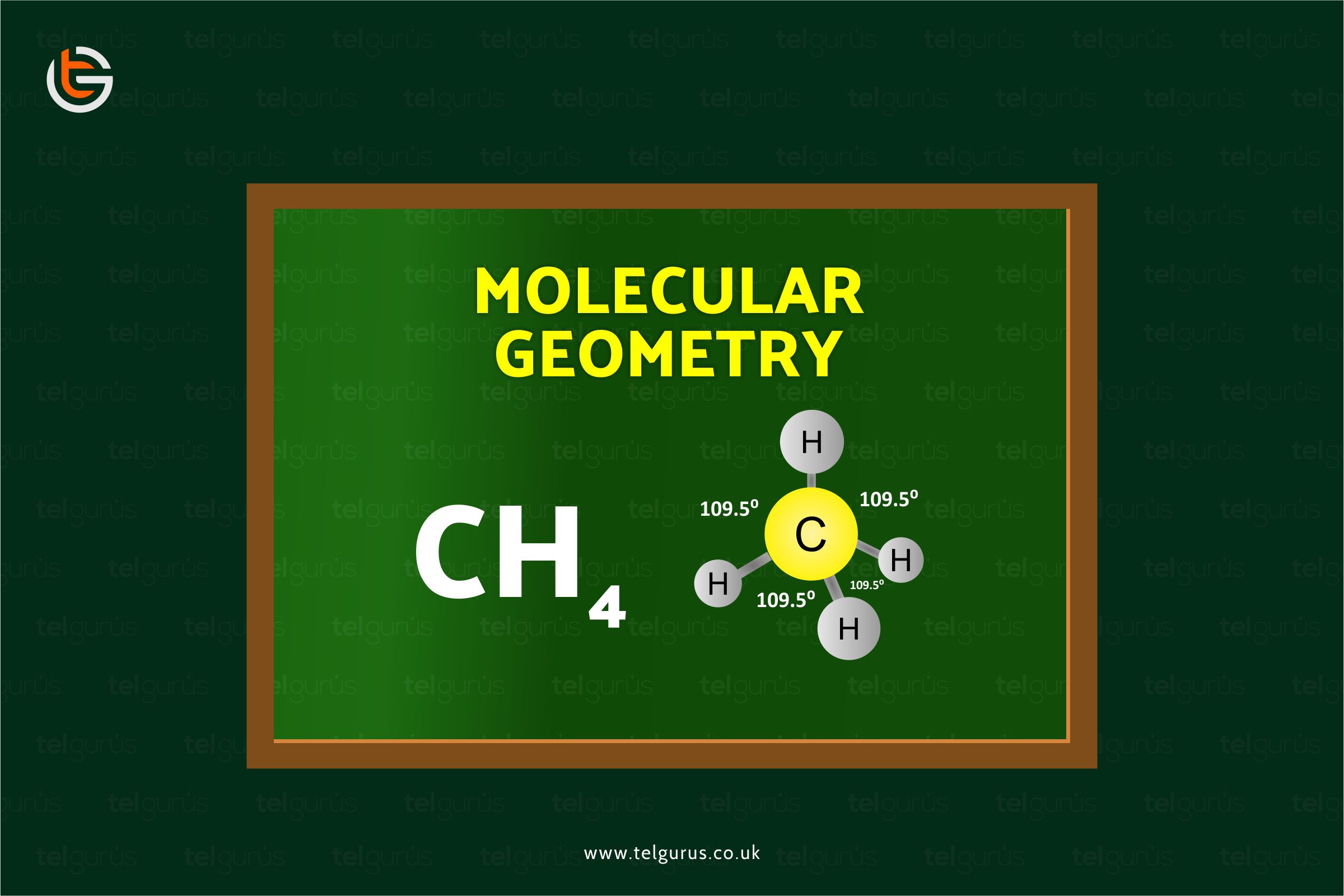
The central carbon atom in the CH4 forms the covalent bond with these four hydrogen atoms, and this sharing generally completes the outer shell of both hydrogen and carbon atoms.
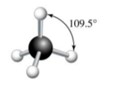
The four hydrogens around the central carbon indicate that there are a total of four electron groups around this carbon atom, generating a molecule’s tetrahedral shape.
These tetrahedral-shaped molecules form the 109 degrees’ bond angles.
Structure of Methane
Methane has a tetrahedral structure comprising four equivalent C-H bonds.
The shape of methane is a regular tetrahedron with carbon at the centre and hydrogen at each corner.
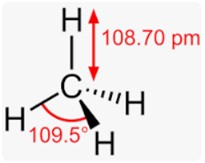
Each H-C-H in methane has an angle of 109.5°, and every CH bond distance is at 1.09 angstroms.
Is CH4 polar or nonpolar?
The methane structure has all the outer atoms the same, that is, it includes the same dipoles, and also the dipole moments are also in the same direction.
The dipole moment is towards the carbon atoms, so the overall molecule becomes non-polar.
Therefore methane has non-polar bonds and is considered non-polar overall.
Why is CH4 tetrahedral in shape?
Methane is considered tetrahedral in shape as it has 4 electron density regions around the central carbon atoms having four bonds and no lone pairs.
And therefore, the resulting shape comes as a regular tetrahedron with H-C-H angles of 109.5°.
Why is the CH4 molecule tetrahedral?
CH4 molecule is tetrahedral as the four C-H bonds in methane are held at an angle of 109 degrees – 28’.
Since the angle in the space at which the repulsions between the four shared electrons pairs is minimum.
Uses of CH4
Methane is used
- In oven, automobiles and water heater
- In electricity generation
- As a rocket fuel
- To sanitize the products
- As an antifreeze ingredient
- In gas-fired power stations
- In the gas appliances testing
- In gas cookers
Bottom Line!
Methane has a tetrahedral molecular structure with carbon at the centre and hydrogen at each corner. We hope this answer has given you clarity over the molecular structure of CH4.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?