Enrich your knowledge with our informative blogs
Describe why diamond is hard and Graphite is soft?
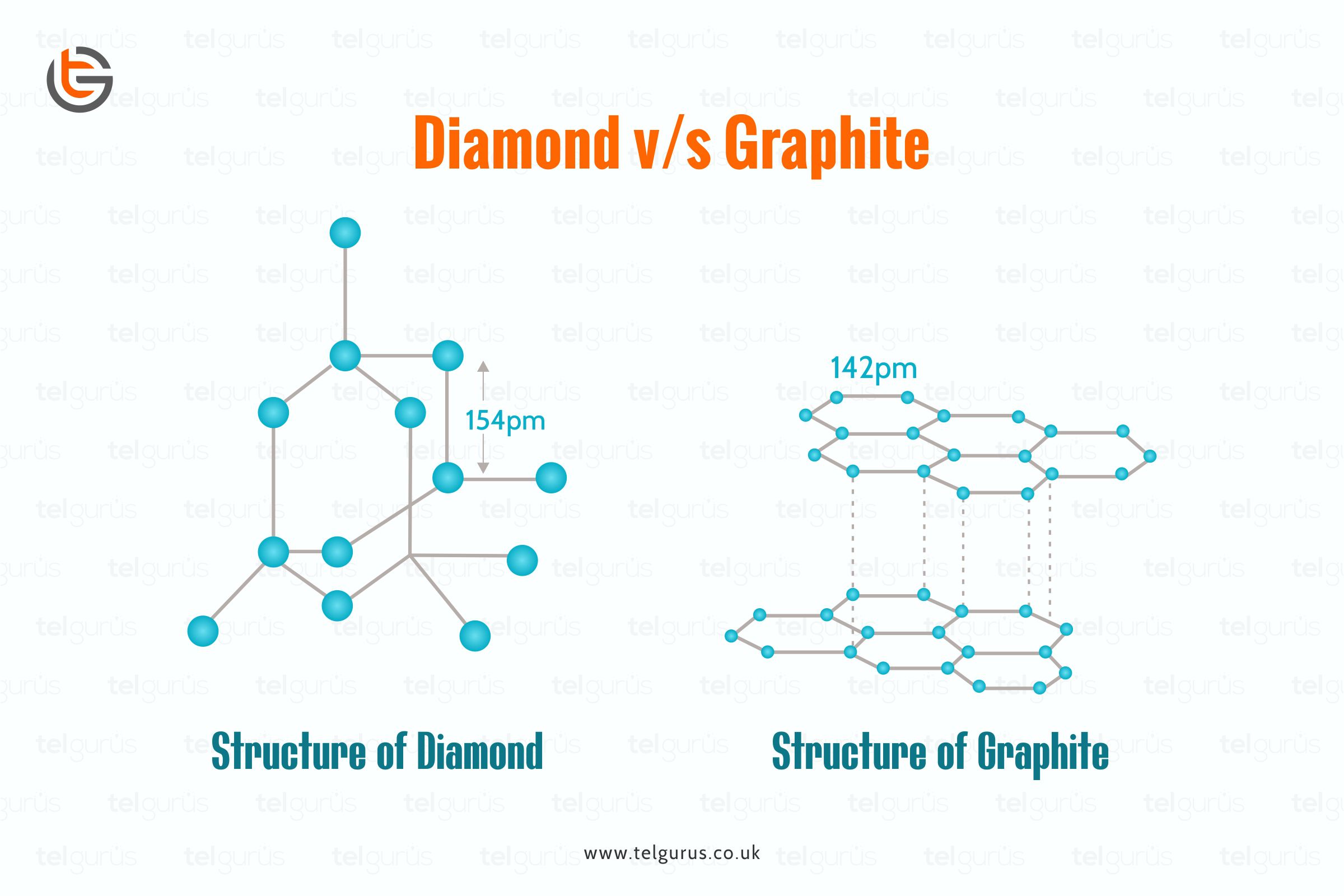
Even though Graphite and diamond are made of carbon, the diamond is hard, whereas the Graphite is soft.
This is due to the strong covalent bond among its atoms forming a regular tetrahedron that is difficult to break.
On the contrary, the Graphite comprises of a flat hexagonal ring forming the carbon atoms layers. The several layers of carbon atoms are so far away from each other that covalent bond formation cannot even exist.
These carbon atom layers are held together by the weak van der Waals forces, allowing them to slide over each other.
And because of this layered structure of carbon atoms, Graphite is stronger than diamond.
Take a rotating graphic of graphite molecules and a diamond structure and try this to understand the reason.
- For Graphite
Rotate the graphite molecule. As the Graphite is layered, and there are strong covalent bonds between the carbon atoms present in each layer, only weak forces exist between layers. This permits the carbon layers to slide over each other.
- For Diamond
Rotate the structure of the diamond, and do not forget that each diamond atom is at the same distance from each of its neighbouring carbon atoms.
There exists a rigid bonds network within the diamond crystal. And in this rigid network, the atoms cannot even move, which is why diamonds are hard.
Bottom Line!
Diamond has a 3-dimensional strong covalent bonds network, and it is very complicated to break the extended covalent bonding. And that is why the diamond is considered the hardest substance.
On the contrary, Graphite has layered structures that are held together by weak Vander Was forces. Therefore, Graphite cleaves easily between these layers, which is why they are soft and slippery.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?