Enrich your knowledge with our informative blogs
Describe why diamond is hard and graphite is soft?

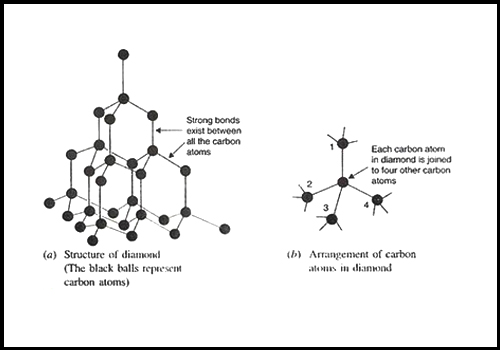
Diamond and Graphite, both are formed from carbon atoms. They possess, unlike structural forms and properties because of the nature of bonds formed amongst Carbon atoms.
Diamond has 4 valence electrons of Carbon atoms which forms a covalent bond with adjoining Carbon atoms. These covalent bonds give strength to a Diamond. The electrons of a Carbon atom are arranged in an sp3 hybridization state.
Book Your 60-minutes Free Trial class NOW!
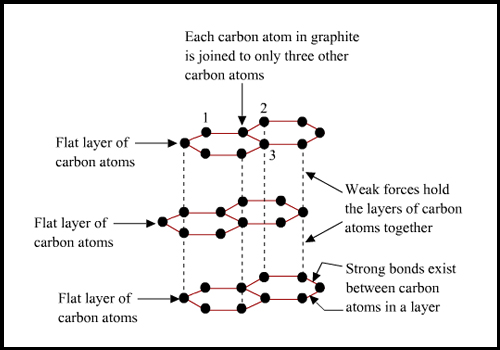
Graphite has 3 valence electrons of carbon atoms that form a covalent bond with adjoining Carbon atoms. The electrons of a Carbon atom are arranged in an sp2 hybridization state. The fourth valence electron, which is weakly bonded to the three neighboring Carbon atoms, is totally free to move which makes the graphite a good conductor of electricity. The planes of graphite are held by weak Van der Waals forces that allow them to slide over each other making graphite soft.

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?