Enrich your knowledge with our informative blogs
What Is OH In Chemistry?
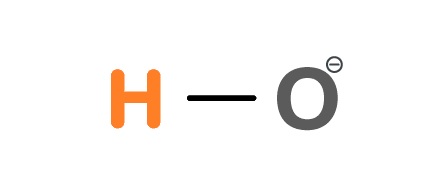
OH- is a diatomic anion that has the chemical name Hydroxide. Hydroxide is also known as the Hydroxide ion, or Hydroxyl radical, or Hydroxyl. It is composed of Hydrogen and Oxygen atom that are held together by a covalent bond.
Hydrogen carries a negative electric charge. It is widely used as a food preservative as an anticoagulant for stored blood and buffer and for alkalization of urine to prevent kidney stones. It acts as a catalyst, a base, a ligand, and a nucleophile.
In this compound, the two electrons share oxygen bonds with Hydrogen. The Hydroxide is negatively charged as an electron has been absorbed.
Structure of Hydroxide
Hydroxide Properties
The ion generates salt where some of them dissociate in an aqueous solution in order to release the solvated hydroxide ions. When Hydroxide and a strongly electropositive center are attached to each other, Hydroxide may ionize itself to release hydrogen cation and make the parent compound an acid.
HO•, an electrically neutral compound, is hydroxyl radical.
–OH, a covalently bound group is the hydroxyl group.
The hydroxyl ion and hydroxyl groups are nucleophiles and function as a catalyst in organic chemistry.
Uses of Hydroxide
- Hydroxide is utilized to produce fuel cells.
- It is used to make disinfectants.
- It is used as a food preservative to prevent bacteria and mold from growing in food.
- It is used in the paper recycling process.
- It is used in the extraction of alumina.
Book Your 60-minutes Free Trial class NOW!
Read More – Chemistry Questions

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?