Enrich your knowledge with our informative blogs
What is the difference between evaporation and boiling?
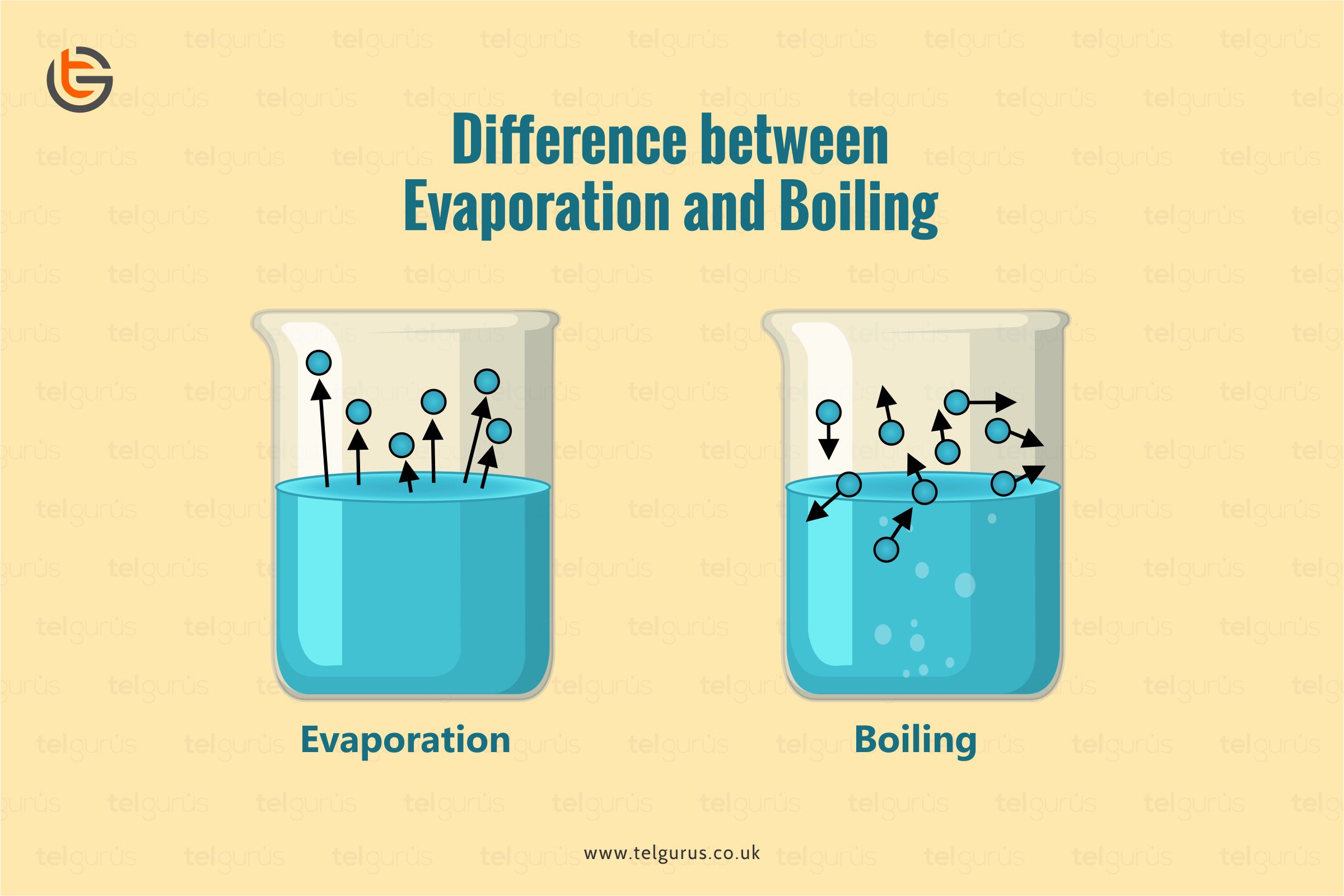
Both the terms boiling and evaporation include changing a liquid form into a gas, but there are several differences between them. Let us get acquainted with the differences between both terms.
What is evaporation?
Evaporation refers to a process where the liquid form gets transformed to the vapor form. Let us take a simple example of evaporation and understand the process.
The evaporation process takes place when water gets evaporated from the soil by the sun’s heating effect. Evaporation takes place at any temperature and doesn’t produce bubbles.
In this process, the molecules or atoms in the liquid state gain adequate energy to transform into the gaseous state.
Therefore, it proceeds more quickly at high temperatures and high flow rates between liquid and gaseous phases in liquids having lower surface tension. So evaporation is considered a physical change.
For example, when you leave a water glass on a table for long enough, you will see that the water level will go down devoid of any interference from the side.
Factors affecting evaporation
- Surface Area
Evaporation is basically a surface phenomenon, and if the surface area is augmented, the evaporation rate also increases.
For instance, we generally spread clothes to dry them faster. So the relation between evaporation rate and surface area can be written as
Rate of Evaporation ∝ Surface area
- Humidity
Humidity refers to the water vapor amount present in the air. Air cannot hold more than a definite water vapor amount at a given temperature. In case the water amount in the air is high, then the evaporation rate decreases.
The relation between evaporation rate and surface area can be written as
Rate of Evaporation ∝1/Humidity
- Temperature
Evaporation rate increases at higher temperatures. With the increase in temperature, more particles get enough kinetic energy to go into the vapor state.
For instance, wet clothes dry rapidly under sunlight. The relation between evaporation rate and temperature can be written as
Rate of Evaporation ∝ Temperature
- Wind Speed
With the augment in the wind speed, the water vapor particles move away with the wind while decreasing the water vapor amount in the surrounding area. Therefore, an increase in the wind speed augments the evaporation rate.
The relation between evaporation rate and surface area can be written as
Rate of Evaporation ∝ Wind Speed
What is Boiling?
Boiling refers to a process of heating up the liquid where the liquid’s temperature is higher than a substance’s boiling point.
Boiling produces bubbles, and most liquids have a boiling point that results in more agitated and rapid movement within particles of a substance.
Here intense heat is added to the liquid molecules that begin moving rapidly throughout the substance.
Boiling can be determined when a liquid transcends the boiling point while leading to bubble formation. This states that a matter’s liquid state has entered into gaseous molecules.
Factors affecting Boiling Point
- Impurities
A compound’s boiling point is utilized as a reference property of it in a pure form.
For instance, pure water boils at 100℃, whereas water with several other substances boils at a higher temperature. Therefore it can be said that impurities in a pure substance lift up the boiling point.
- Atmospheric Pressure
A substance’s boiling point modifies according to the pressure of its surrounding. At higher pressure, more energy is necessary to rupture the bonds between particles resulting in the augment of a boiling point.
Therefore the higher pressure boosts the boiling point, whereas the lower pressure depresses the boiling point.
Understanding the difference between evaporation and boiling
| Parameters | Evaporation | Boiling |
| Process | Evaporation refers to a normal process that takes place when the liquid form changes to a gaseous form while leading to an increase in pressure or temperature. | Boiling refers to an unnatural process in which liquid gets heated and vaporized because of the continuous heating of the liquid. |
| Occurrence surface | It usually takes place on the liquid’s surface is heated up. | It takes place on the liquid’s entire mass that gets heated up. |
| Bubbling | The bubbling effect is not visible here. | The bubbling effect is visible here during the boiling process. |
| Speed | The evaporation process is slower and more carried out. | The process is much quicker and takes place rapidly. |
| Temperature required | A liquid evaporates at any temperature above freezing. | Boiling occurs only when the liquid reaches a specific temperature. |
| Source of energy | Evaporation utilizes the energy that is already present in the liquid. | Boiling requires external energy sources like a burner. |
| Time | It takes a longer time to complete | It takes a shorter period |
| Energy | It needs little or no energy | A lot of energy adds to the process |
Bottom Line!
The primary difference noticed between both the terms is in their occurrence place where evaporation occurs on the surface of a liquid. In contrast, Boiling occurs over liquids with a large mass.
The rest differences are explained above. We hope that it gave you a clear idea about the dissimilarities.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?