Enrich your knowledge with our informative blogs
What is the overall charge of the nucleus of an atom, and why?
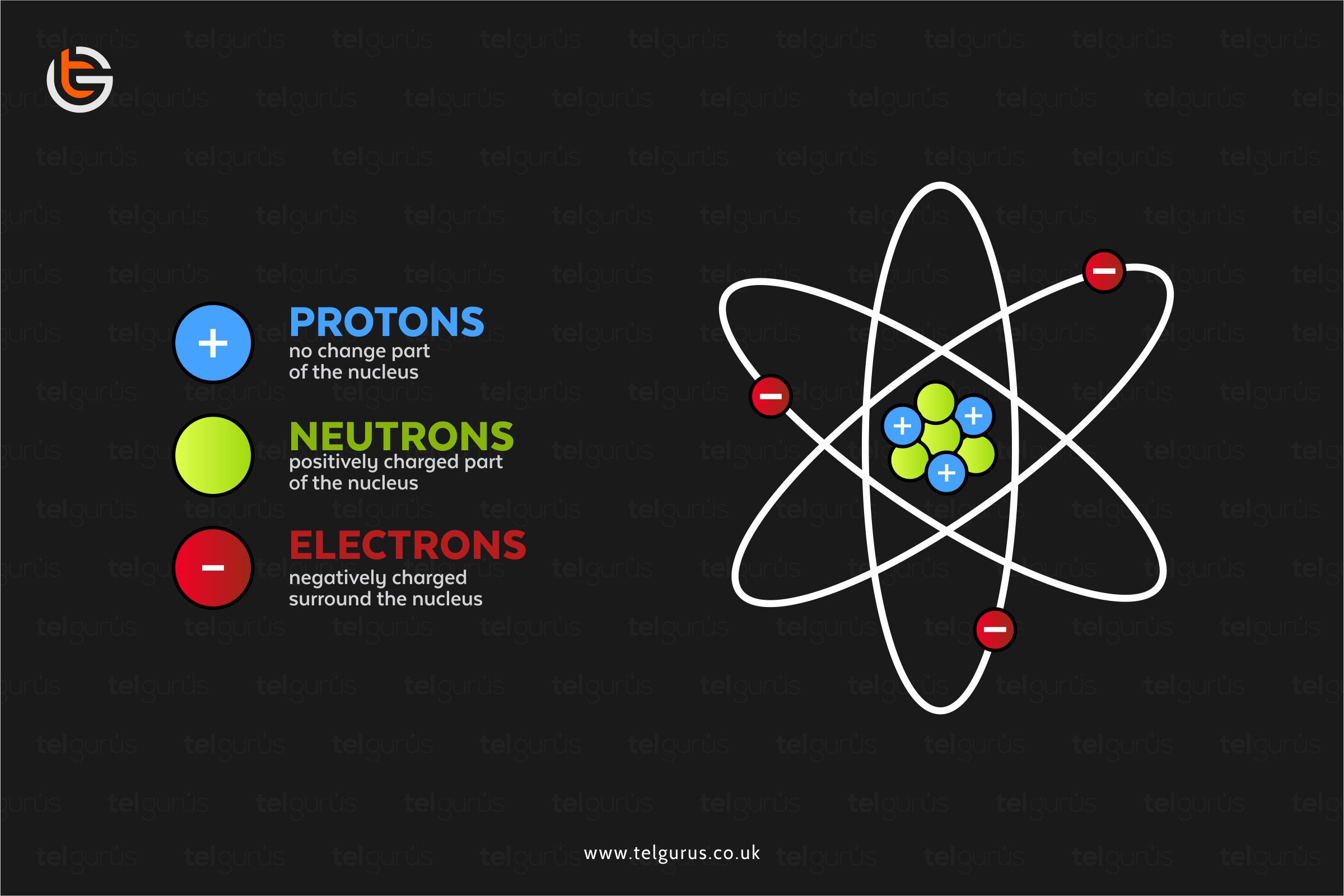
An atom comprises a tiny nucleus surrounded by moving electrons. And nucleus is basically a collection of neutrons and protons where
- Neutrons are electrically neutral
- And Protons are positively charged
So, what is the overall charge of the nucleus of an atom?
A nucleus is consists of the neutrons and protons where
- Neutrons have a neutral charge
- And Protons have a positive charge
A neutral charge won’t cancel out the positive charge that indicates that the overall charge of a nucleus is positive.
And why is the overall charge of a nucleus Positive?
The neutrons present in the nucleus have a neutral charge, whereas the protons in the nucleus have a positive charge. As neutral won’t cancel out the positive charge, so the overall charge of the nucleus is positive.
What determines the positive charge of the nucleus?
The number of protons present in the nucleus determines the positive charge of the nucleus.
As the protons are positively charged, and a nucleus comprises protons and neutrons, so the protons present in the nucleus determine the positive charge.
Even though the nucleus contains neutrons also, they do not impact the charge as they have no charge, and therefore they do not contribute to the nucleus charge.
Bottom Line!
A combination of neutral particles and positively charged particles definitely forms a positively charged particle only.
Therefore, a nucleus has an overall positive charge.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?