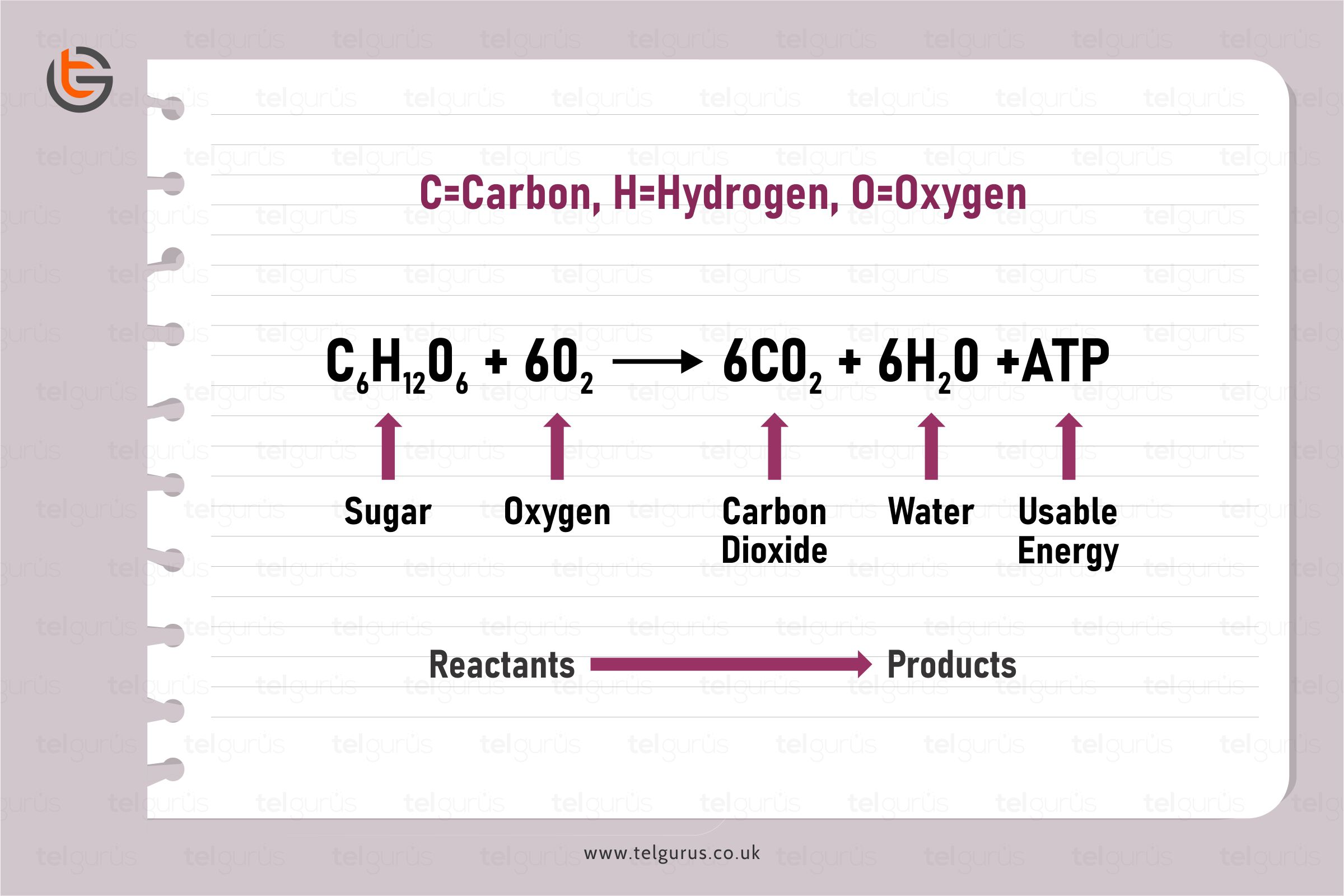
Enrich your knowledge with our informative blogs
Balance the following equation: C3H8 + O2 —-> CO2 + H2O.
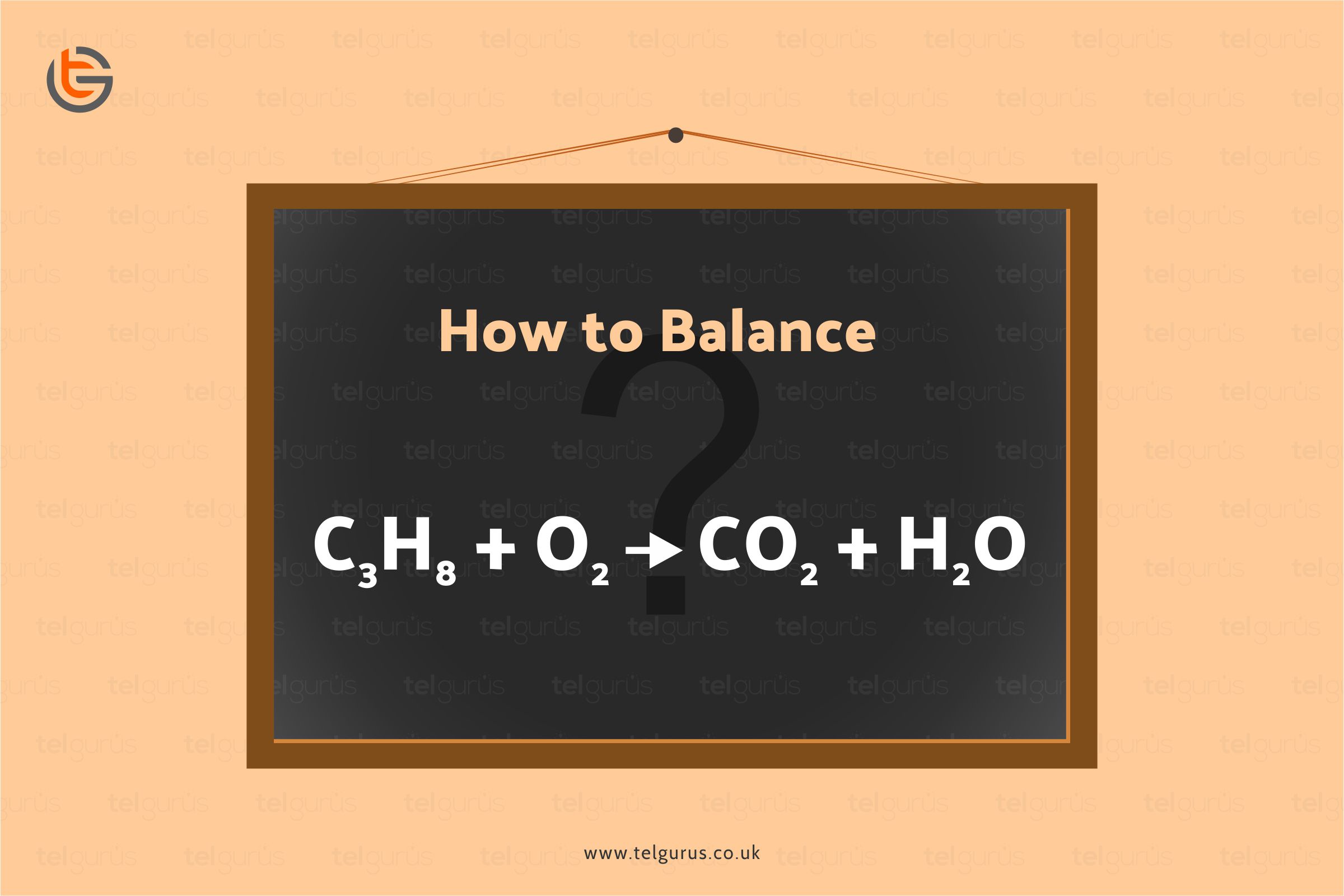
C3H8 + O2 ——-> CO2 + H2O
Balancing the equation
Comparing the number of reactants atoms with the number of products atoms.
First of all, list the number of each atom for the product and reactant, both sides of the equation.
Here in this example, listing the number of reactants and products can be written as
On the reactants side On the product side
Carbon: 3 Carbon: 1
Hydrogen: 8 Hydrogen: 2
Oxygen: 2 Oxygen: 3
Writing the resultant equation
Now, after this, the first step to balance the number of carbon atoms is by multiplying the CO2 on the product side by 3. Therefore, the new equation will be then written as
C3H8 +O2 ——–> 3CO2 + H2O
As the carbon atoms are now balanced on each side, we will move further to balance the number of Hydrogen atoms.
The Hydrogen atoms at the reactant side are eight, whereas on the product side are two only, so multiplying the hydrogen on the product side will balance the number of the hydrogen atom. It makes sense, right?
Book Your 60-minutes Free Trial class NOW!
Let us see multiplying four on the product side will make what sort of difference. Writing the resultant equation,
C3H8 + O2 —–> 3CO2 + 4H2O
Now we have eight hydrogen atoms on each side, as we are left with Oxygen atoms only to balance; let us see how we can attain that.
As there are 10 Oxygen atoms on the product side and only two on the reactants side. So, we need to balance that as well, and therefore, multiplying the O2 atoms with five will make 10 Oxygen atoms overall on the reactant side. Let us move further with the balancing of the equation.
Multiplying 5 with O2 on the reactant side and writing the resultant equation
C3H8 + 5O2 ——–> 3CO2 + 4H2O
So, according to us, this is our balanced equation! Let us compare the number of atoms on each side.
On the Reactant side On the Product side
Carbon: 3 Carbon: 3
Hydrogen: 8 Hydrogen: 8
Oxygen: 10 Oxygen: 10
As the number of atoms on the products and reactant sides is equal, we can say that the equation is balanced.
Book Your 60-minutes Free Trial class NOW!
Bottom Line!
C3H8 + 5O2 ——–> 3CO2 + 4H2O is the final balanced equation of the given equation.
Don’t forget to try the same method to balance your other equations.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?