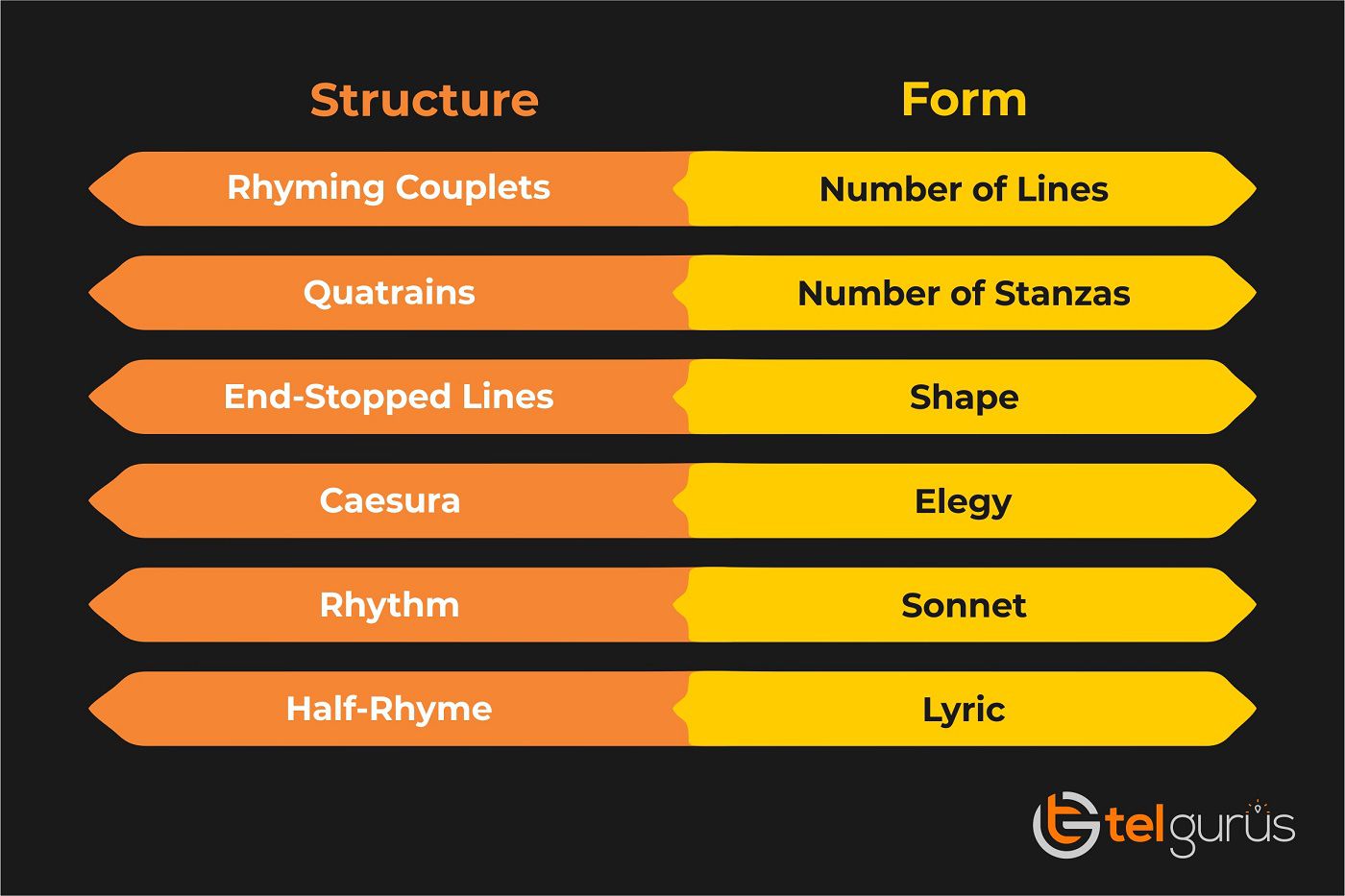
Enrich your knowledge with our informative blogs
Describe How Crude Oil Is Separated Into Fractions?
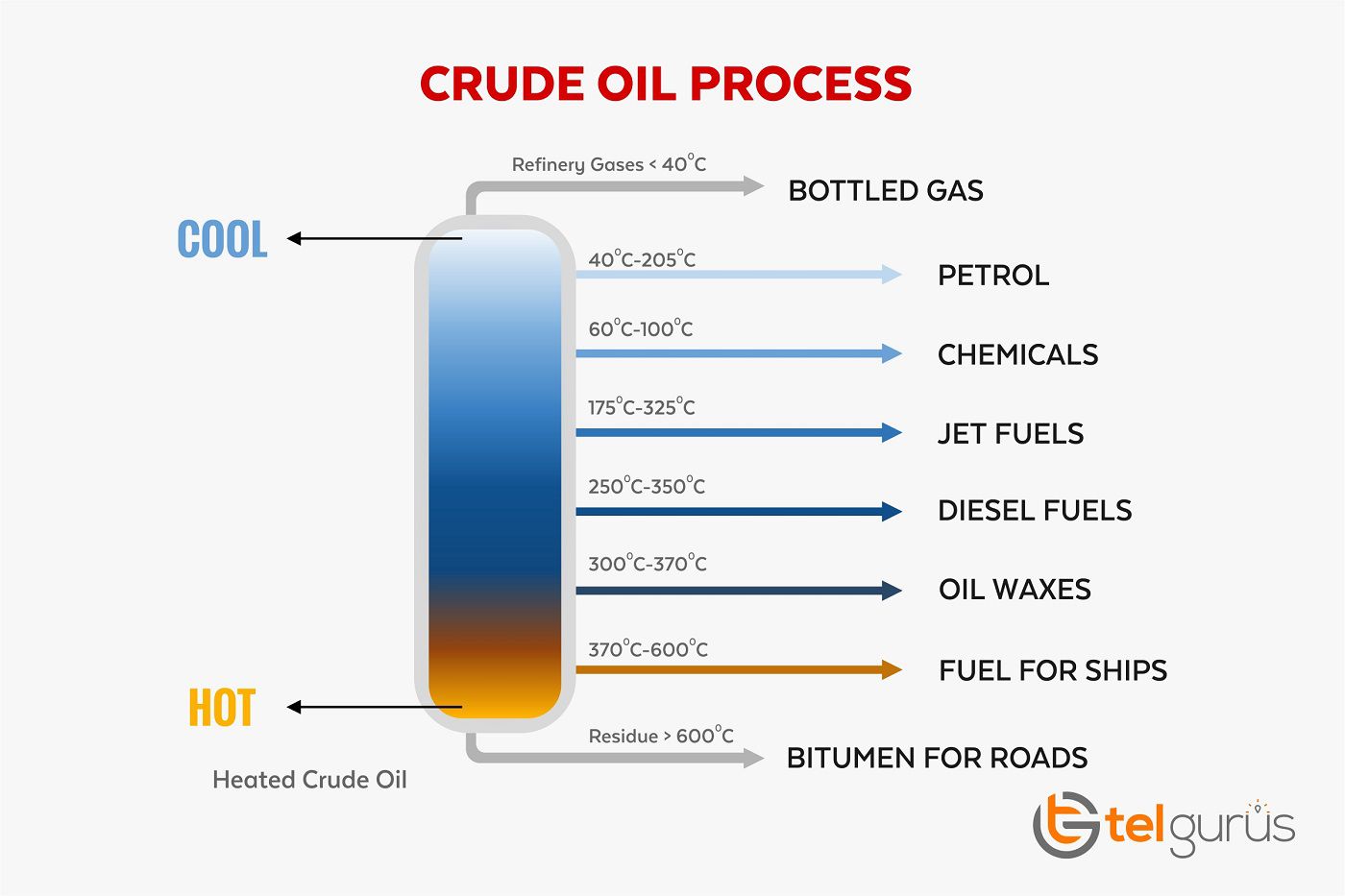
Crude oil is a restricted asset. Fracture is used to transform lengthy alkanes into small, more functional hydrocarbons. The fractional distillation procedure is the detachment of a mixture into its component parts. Basically, is the procedure of split crude oil into groups of hydrocarbons with the related numeral of carbon atoms. We designate this category of hydrocarbons “fractions”.
When heat is an appeal to the crude oil chemical compounds are split – bring about the fractions of the combination to vaporize and distil. A selection of crude oil is a category of hydrocarbon molecules of related size with alike boiling tips. There are many ways to identifying the useful fractions which are distilled from crude oil.
Book Your 60-minutes Free Trial class NOW!
One common method is by fractionating into three types: light, middle, and substantial fractions. Heavier components condense at higher temperatures & these are removed at the bottom of the column. An oil cleanser cleans and breaks up the crude oil into numerous fuels and by-products. The most supreme one is gasoline. The other petroleum outcome is diesel fuel, heating oil, and jet fuel.
We can sum up the fractional distillation process into 3 parts.
A) Evaporation
- Crude oil is warmed up till it evaporates.
- Oil vapour is admitted a fractionating pillar at the foundation and stands up skywards.
B) Condensation
- The warmth is towering at the foundation of the column. Long-series hydrocarbons precipitate at the foundation and are composed as liquids.
- Short-series hydrocarbons have foundation boiling points. They constrain the pillar and precipitate at lower temperatures nearby the top.
C) Collection
- The fragment is collected. They are then prepared to generate end products:
- Fuels are an ordinary product.
- Petrochemical production can use some fragments as feedstock to make solvents, lubricants, detergents, etc.
Book Your 60-minutes Free Trial class NOW!
The table appears some knowledge about four of the fragments from crude oil that are used as fuels.
| Fragments | Boiling point in °C | Numeral of carbon atoms initiate in the molecules |
| Gasoline (petrol) | 20-200 | 5-10 |
| Kerosene (paraffin) | 180-260 | 10-16 |
| Diesel | 260-340 | 14-20 |
| Fuel oil | 370-600 | 20-70 |

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?