Enrich your knowledge with our informative blogs
Explain how pressure can affect the rate of reaction?
The rate of reaction refers to the speed at which the reactants are actually converted to products. While talking about the chemical reactions, some reactions are instant and immediately produce the products, whereas others take time to reach the final equilibrium.
How does pressure affect the rate of reaction?
Pressure increases the gases concentration resulting in the increase of the rate of reaction. The reaction rate decreases in the reverse direction and increases in less gaseous molecules direction. Therefore it is clearly understood that Concentration and Pressure are interlinked, and both of them affect the rate of reaction.
What exactly happens?
The increase in the pressure on a reaction involves the reacting gases increasing the rate of reaction. And modifying the pressure on a reaction involving solids or liquids only has no effect on the reaction rate.
For instance,
In the case of manufacturing ammonia using the Haber Process, the reaction rate between nitrogen and hydrogen is increased using very high pressures.
H2 (g) + 3H2 (g) ⇌ 2NH3 (g)
And the primary reason to use the high pressures is to enhance the ammonia percentage in the equilibrium mixture, but with this, there is a useful impact on the reaction rate also.
And here comes the explanation for the same
Relationship between Concentration and pressure
Increasing a gas’s pressure is similar to increasing its Concentration. If you are given a mass of gas, the way to increase the pressure is by squeezing it into a smaller volume. And in the case of the same mass in a smaller volume, then the Concentration is higher.
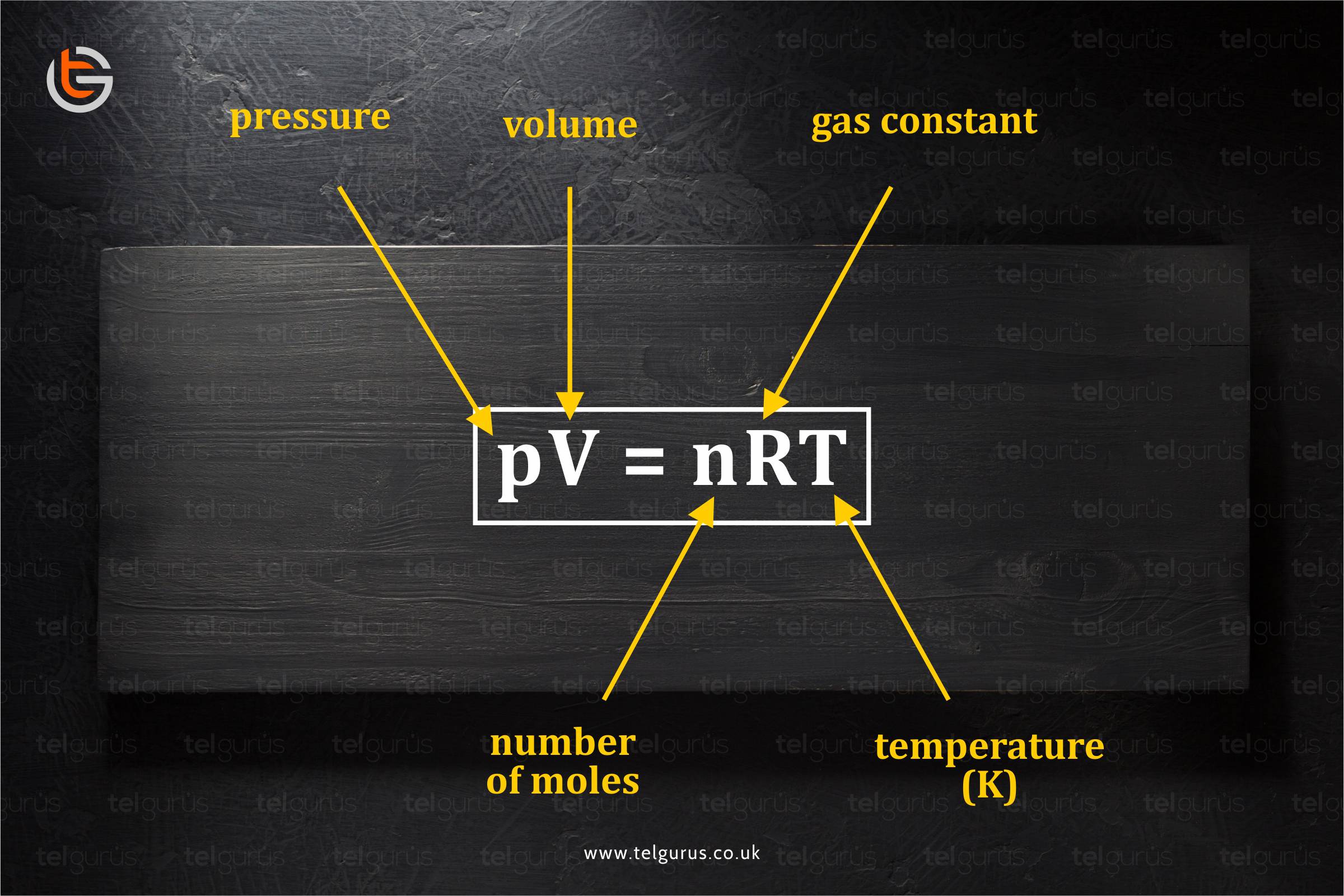
While mathematically showing the equation, the ideal gas equation becomes,
pV = nRT
where
- p is pressure
- v is the volume
- n is the number of moles
- R is gas constant
- T is the temperature
Now, rearranging the equation
P = n/V X RT
Where
- p is pressure
- n/V is the number of moles divided by volume is Concentration
- RT is constant at a constant temperature
As RT is constant, as long as the temperature remains constant, the pressure is directly proportional to Concentration. And if you double one, the other will also get doubled.
Effect of increasing pressure on reaction rate
Talking the two particles, where the reaction involves either the collision between two diverse particles or two similar particles. For any reaction to take place, the particles must first collide. Whether both the particles are in a gaseous state, or one is solid, and the other is gas, if the pressure is increased, the chances of collision also increase.
When the reactions involve a single particle, things get way complicated as the reactions involve something taking place to a single particle only rather than being generated by the collision between two different particles.
This case works well for the gaseous reactants as they are highly compressible. However, changing pressure for a reaction involving only liquids or solids has no such effect.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?