Enrich your knowledge with our informative blogs
Explain the process of Osmosis and give an example of where this occurs?
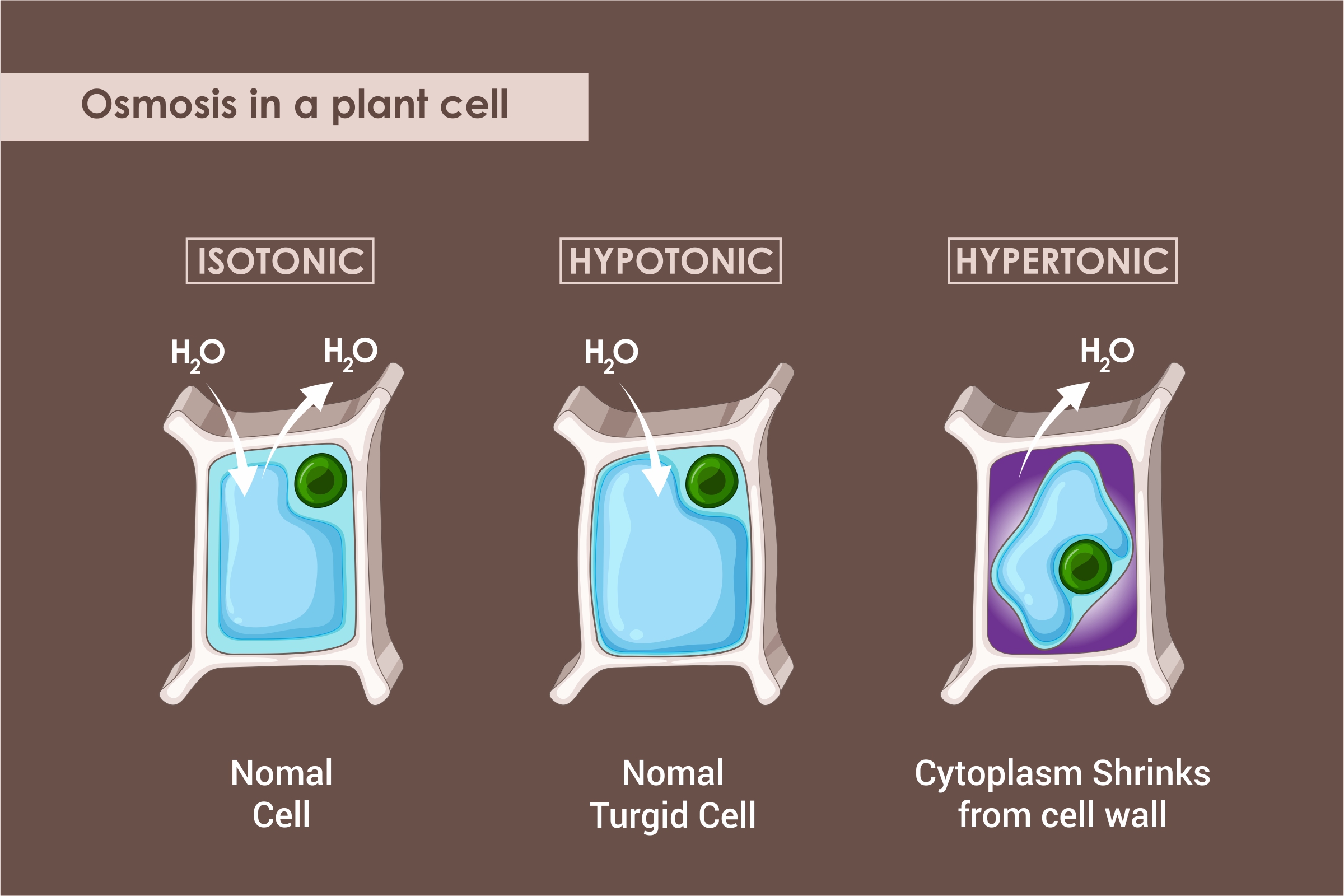
Osmosis refers to a process by which a solvent’s molecules pass from a low concentration solution to a high concentration solution through a semi permeable membrane.
What is Osmosis?
Osmosis is a passive process that occurs devoid of any energy expenditure. Osmosis involves the movement of the molecules from high to low concentration regions until the concentrations become equal on either side of the membrane. Any of the solvents can undergo the osmosis process, including supercritical liquids and gases.
Osmotic Solutions
Osmotic solutions are of three different types. This includes:
- Isotonic Solution
An isotonic solution has the same solute’s concentration both outside and inside the cell.
- Hypotonic Solution
A hypotonic solution has a higher concentration of solute inside the cell as compared to outside.
- Hypertonic Solution
A hypertonic solution has a higher concentration of solute outside the cell as compared to inside.
Osmosis Types
Osmosis is of two types. This includes:
- Exosmosis
Whenever a substance is placed in a hypertonic solution, the molecules of solvent move outside the cell, and the cell undergoes plasmolysis or becomes flaccid. This process is known as exosmosis.
- Endosmosis
Whenever a substance is placed in a hypotonic solution, the molecules of the solvent move inside the cell, and the cell undergoes deplasmolysis or becomes turgid. This process is known as endosmosis.
Effects of Osmosis on cells
The osmosis process affects every cell differently. For instance, if we talk about an animal cell, it will lyse when placed in a hypotonic solution compared to a plant cell. A hypotonic solution is considered an ideal solution for a plant cell because it has thick walls and it requires more water, so the cells will not burst even when placed in a hypotonic solution.
While talking about the animal cell, it survives only in an isotonic solution, while the plant cells in the same solution are no longer turgid, and the plant’s leaves droop.
However, the osmotic flow can be reversed or stopped, which is known as reverse Osmosis. This can be done by exerting external pressure on the solute’s sides. And the minimum pressure needed to stop the solvent transfer is known as osmotic pressure.
Example of Osmosis
Osmosis has a significant role to play in animals, plants, and humans. Here are a few examples of Osmosis.
- If a saltwater or freshwater fish is placed in water with varying salt concentrations, the fish dies because of the exit and entry of water in fish cells.
- The water absorption from the soil is because of the osmosis process. As the plant roots have a higher concentration as compared to the soil, so consequently the water flows into the roots.
- Individuals suffering from cholera are also affected by the osmosis process. The bacteria which overpopulate the intestine, reverse the absorption flow and does not allow the intestine to absorb the water, which further results in dehydration.
- The plant’s guard cells are also affected by the osmosis process. This happens when the plant cells are filled with water it results in the swelling up of the guard cells and opens up the stomata.
- Whenever the fingers are placed in water for a longer time, they become pruney because of the water flow inside the cells.
Visualize the in-depth understanding of the natural world!
Biology would sound more interesting when your curiosity levels are satisfied with better visuals & logical explanations.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?