Enrich your knowledge with our informative blogs
Explain what occurs when an acid reacts with an alkali in terms of ions and molecules. Also, show the equation.
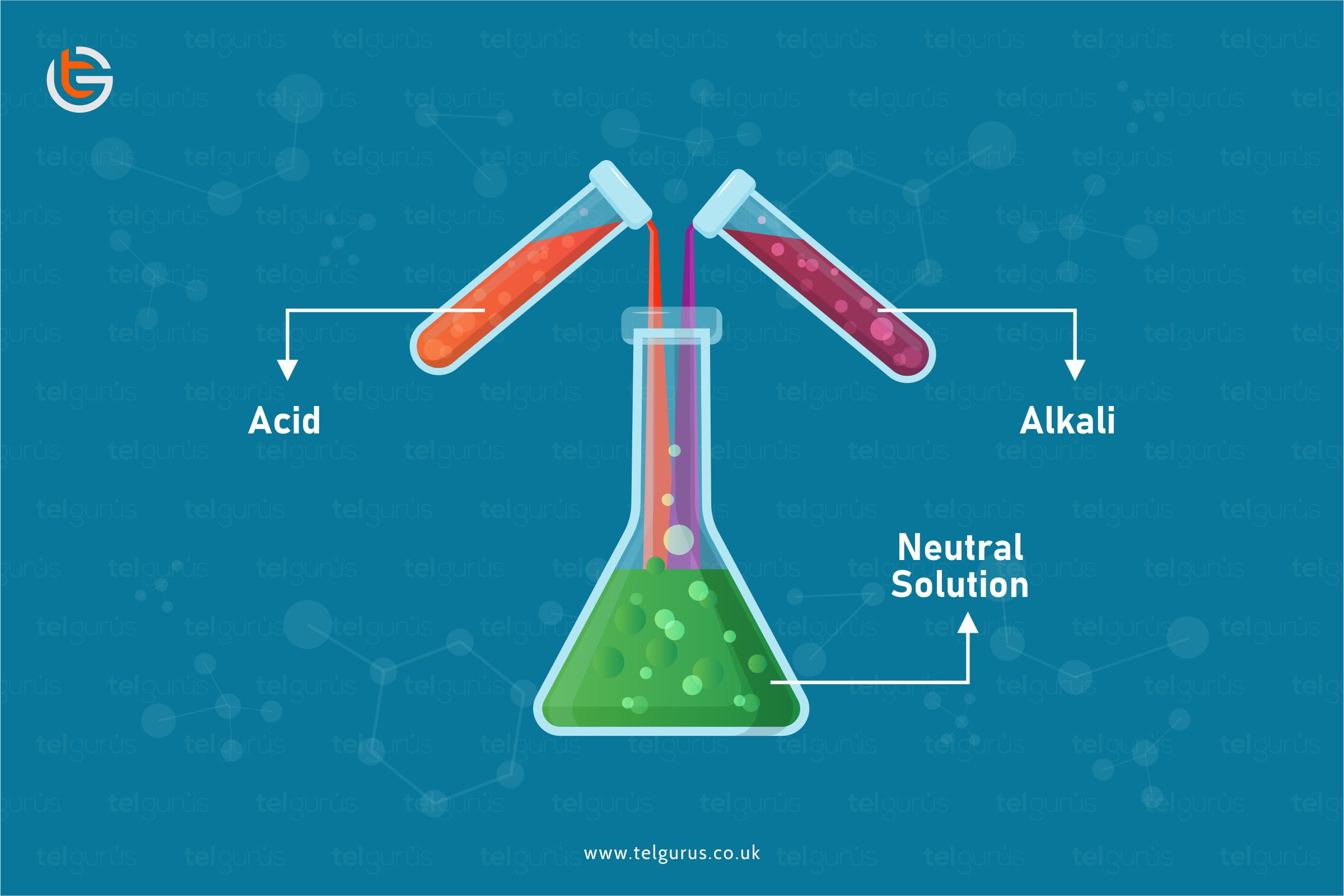
When an acid reacts with an alkali, a chemical reaction happens and the formation of a new substance takes place.
Let us get acquainted with everything you need to know about the query.
- What happens when you react acid with Alkali?
Whenever you add acid with Alkali, a chemical reaction takes place, and a new substance is formed.
If you mix the exact amounts of acid with Alkali, you will end up with a neutral solution which is also known as a neutralization reaction.
- What exactly is it called when an acid reacts with Alkali?
An acid reaction with Alkali or, say, an acid-alkali neutralization refers to a reaction between the hydroxide and hydrogen ions forming water.
- Whenever an acid and Alkali are mixed, what sort of reaction takes place?
Whenever the acid and Alkali are mixed, a neutralization reaction takes place, forming salt and water.
A neutralization reaction is always
Acid + Alkali ——> Salt + Water
Equation of acid reacting with Alkali
H+ + OH- —> H20
However, here the question is about ions, and there is no salt. So, the acids are H+ ions that give them the desired acidic properties, and alkalis are the OH- ions. And together, they react to form water.
Essential to Remember!
- Alkalis in the solution are the sources of Hydroxide ions represented by OH-
- And Acids in the solution are the sources of Hydrogen ions represented by H+.
And so the reaction is represented as
H+ (aq) + OH- (aq) → H2O (l)
Bottom Line!
When it comes to checking the reaction of an acid with alkalis in terms of ions and molecules, the Hydrogen ions from the acid react with the hydroxide ions from alkalis to form water.
And the reaction is represented as
H+ (aq) + OH- (aq) → H2O (l)
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?