Enrich your knowledge with our informative blogs
How do I know if an enthalpy change should be positive or negative?
An enthalpy change refers to the measure of heat absorbed or evolved. Let us get acquainted with everything you need to know about the Enthalpy change.
To better understand the energy change happening during a reaction, you need to understand the two parts of the universe, namely the surroundings and the system.
Surrounding refers to everything in the universe that is generally not a part of a system. The system relates to a matter’s specific position in a particular space studied during an observation or experiment.
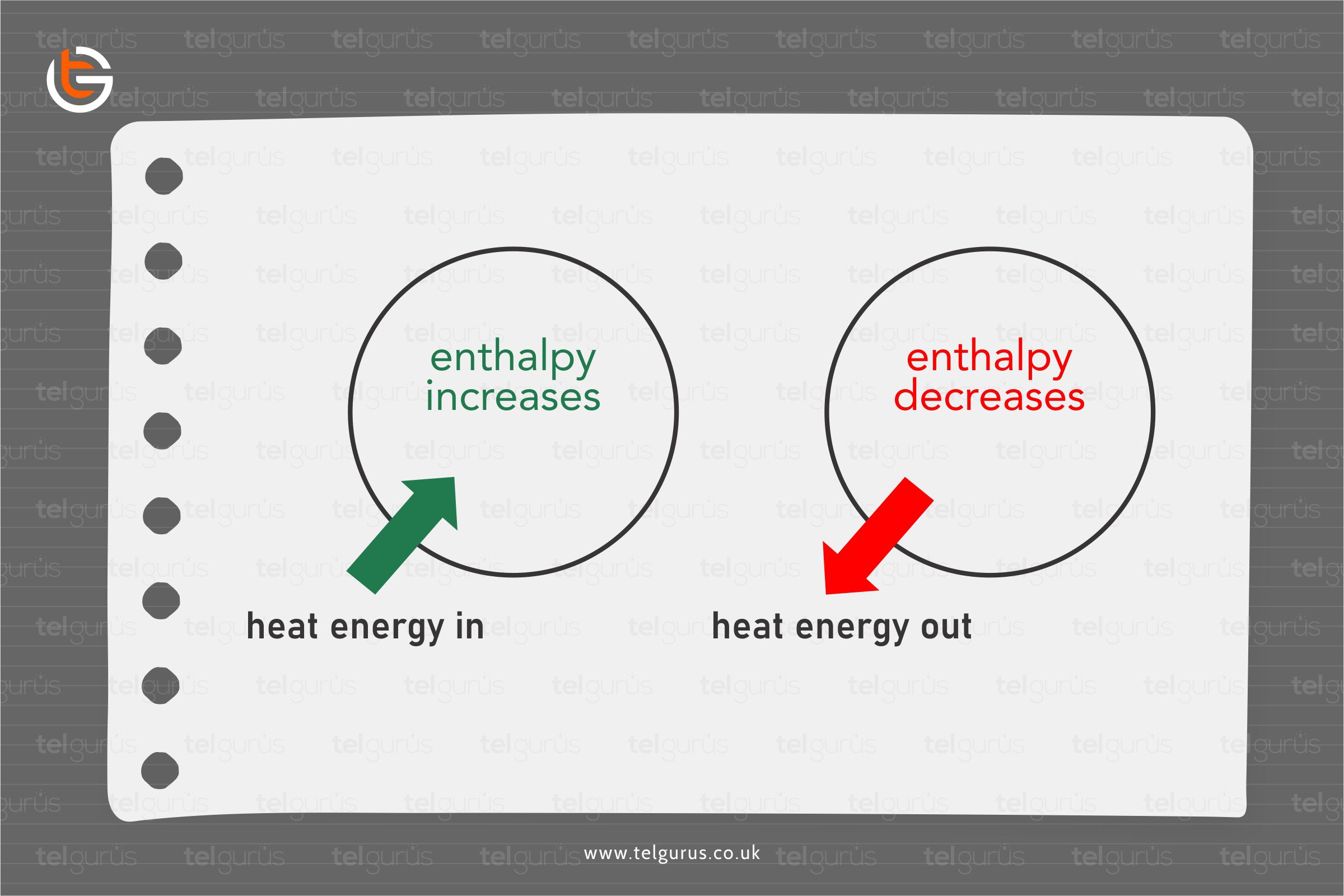
Suppose a system loses a specific amount of energy. In that case, the same amount of energy is generally gained by the surroundings, whereas if a system gains a particular amount of energy, the same energy is supplied to by the surroundings.
- A physical change or a chemical reaction is endothermic if the system from the surroundings absorbs the heat.
- A physical change or chemical reaction is exothermic if the heat is liberated by the system into the surroundings.
What is Enthalpy?
Enthalpy refers to the heat changes in chemical reactions that are often measured in the laboratory under several conditions where the reacting system is open to the atmosphere. It’s a case where the system is at constant pressure.
So, we can say that the Enthalpy represented by H is the heat content of the system at a constant pressure. The changes in Enthalpy of a chemical system are routinely measured as the reactants are converted into products.
The heat released or absorbed by a reaction at constant pressure is similar to the enthalpy changes. It is given by the symbol ΔH.
Enthalpy Change
The enthalpy change of a reaction refers to the measure of differences in the Enthalpy of products and reactants. A system’s Enthalpy is generally identified by the energies required to break the chemical bonds and form chemical bonds.
A Thermochemical Equation
A thermochemical equation includes the enthalpy change of a reaction.
How do I know if the enthalpy change should be negative or positive?
A negative enthalpy change represents an exothermic reaction in which the energy is liberated from the reaction. In contrast, a positive enthalpy change represents an endothermic reaction in which the energy is taken in from the surroundings.
While deciding whether the change should be endothermic or exothermic and calculating the enthalpy change, you need to work on how many bonds are broken and how many bonds are formed.
The bond-breaking procedure requires energy, whereas the formation of the bond releases energy.
This applies to more than just the covalent bonds where attraction forces between the molecules are formed, which also applies to the energy release and vice versa.
In order to work out with the enthalpy change, we just add up the energies lost and gained in the reaction.
For instance, when working on the enthalpy formation of NaCl, when chlorine and sodium ions come together to generate NaCl.
NaCl is the one component of the overall reactions often known as lattice energy that we add up with several other components to identify the overall Enthalpy of formation.
So, should lattice enthalpy be negative and positive?
Bringing together the two oppositely charged ions, generating an ionic bond will liberate energy. It is an exothermic change where the lattice enthalpy must be negative.
When does the Enthalpy change positive or negative?
The enthalpy change ΔH is negative or positive is determined by
- ΔH is negative for the exothermic reactions that evolve heat to the surroundings.
- ΔH is positive for the endothermic reaction that absorbs heat from the surroundings.
Bottom Line!
The enthalpy change is negative or positive that is determined by the type of reaction. The Enthalpy is negative for exothermic reactions but is positive for endothermic reactions.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?