Enrich your knowledge with our informative blogs
Understanding enzyme-substrate function.
Enzymes are the well-known magical proteins essential for life. But how do these enzymes’ function? How do they catalyze only specific biochemical reactions? How do they speed up biochemical reactions so quickly? But before that, let us start with the definition.
What are enzymes?
Enzymes refer to the biological molecules that act as catalysts and aid the complex reactions to take place everywhere in life.
For instance, you ate a piece of meat. Then proteases will go to function and facilitate them breaking down the peptide bonds among the amino acids. So, does that mean that all the enzymes break down all the substances? Well, no, since the enzymes are specific catalysts, so they function to complete one task.
Functioning of enzyme
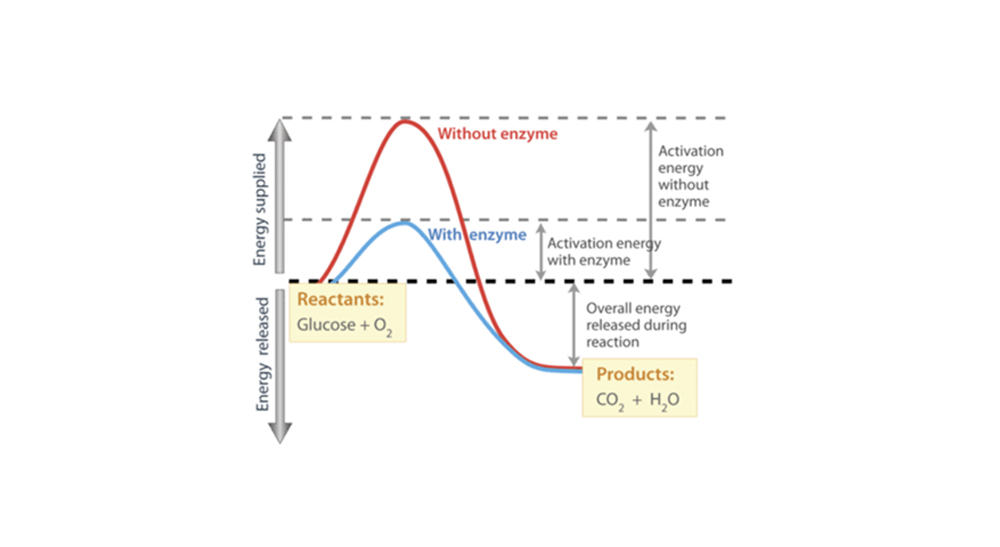
Like all the other catalysts, enzymes function by lowering down the chemical reaction’s activation energy.
A substance that speeds up the chemical reaction devoid of being a reactant is known as a catalyst. The catalysts for the biochemical reactions that occur in living organisms are known as enzymes. And enzymes are proteins.
While considering the enzyme’s functioning, enzymes perform a crucial task of lowering down the activation energy of a reaction that refers to the amount of energy that must be used to put in for a reaction to begin. Activation energy basically refers to the energy required for a chemical reaction.
Enzymes function by binding to reactant molecules and holding them so that the chemical bond-forming and bond-breaking processes take place more readily.
The reaction represented here by this graph is a combustion reaction that involves the reactants Oxygen and Glucose. And the reactants products here are Water and Carbon Dioxide, and energy is also released during it.
The enzymes here speed up the reaction by lowering down the activation energy required to begin. Compare the activation energy levels with and without enzymes.
Enzymes Action
Enzymes bring the reactants together so that they do not have to expend the energy to move until they collide at random.
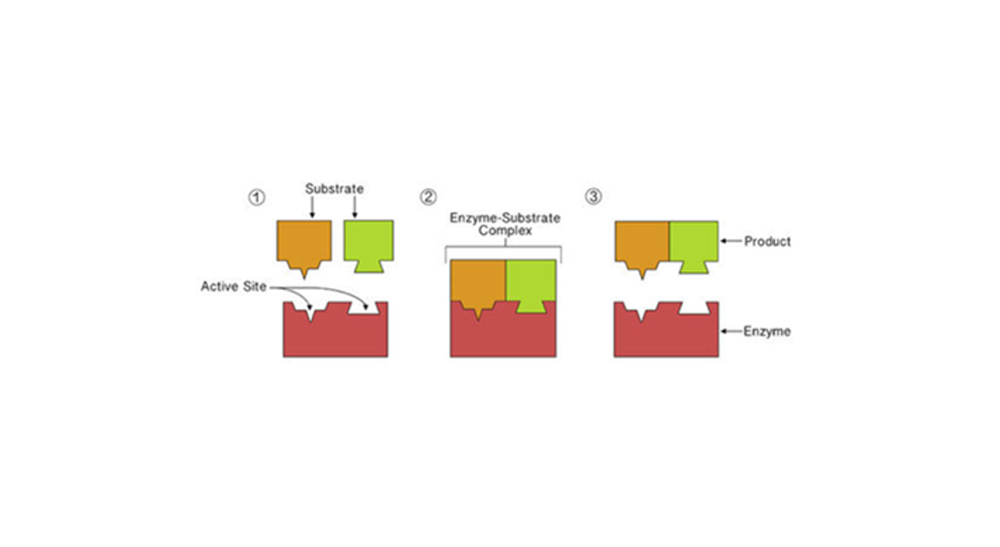
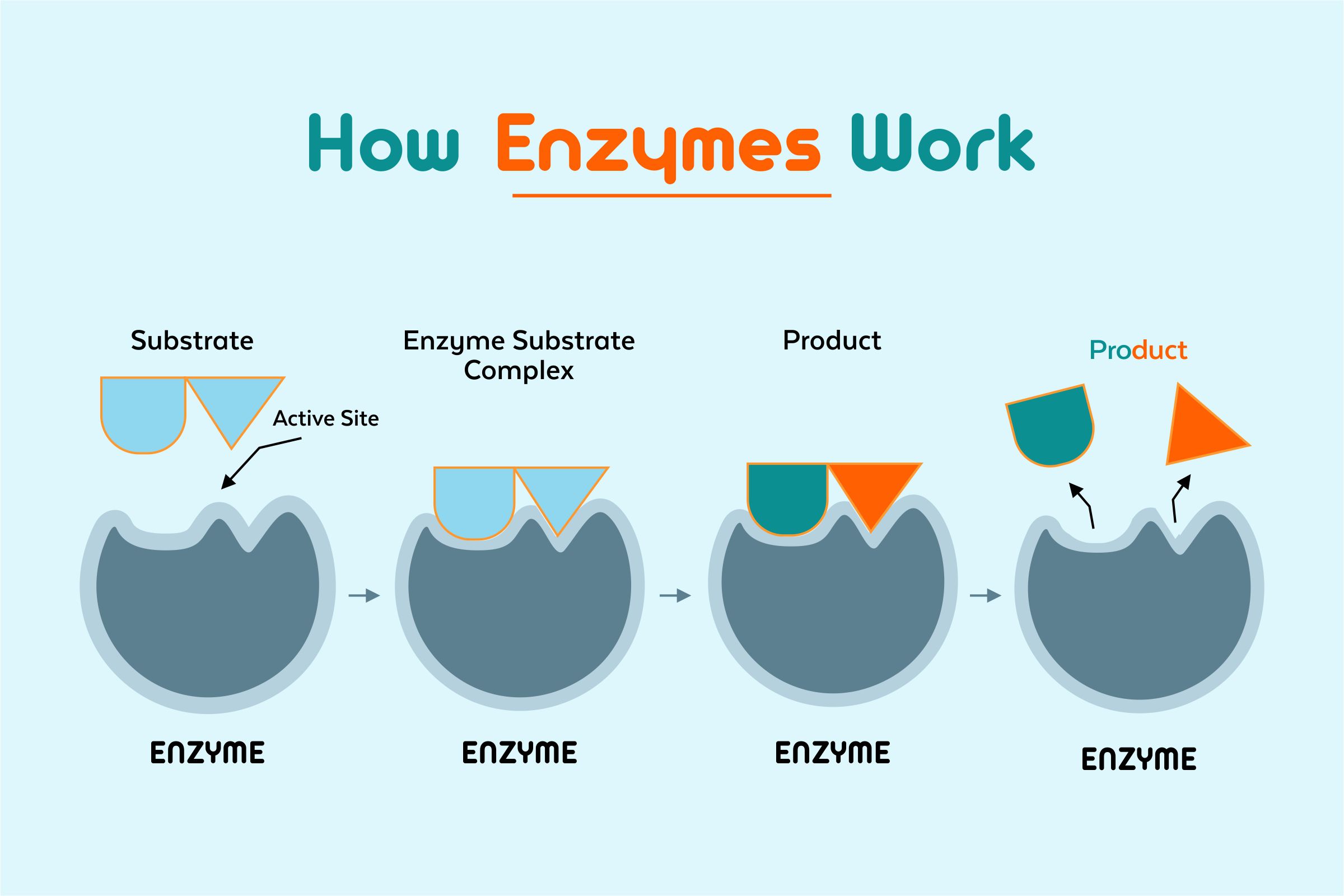
Enzymes bind both the reactant molecules known as substrates at a site on enzyme molecules known as an active site.
By binding the chemical reaction’s reactants at an active site, enzymes also position the reactants appropriately so that they do not have to overcome the intermolecular forces that would push them apart otherwise.
This step enables the molecules to interact even with less energy.
They may also enable the reactions to take place in diverse pathways that have lower activation energy levels.
Here in the below-mentioned diagram, the active site is specific for biochemical reaction’s reactants, the enzyme catalysts, and just like the puzzling pieces fitting together, the active site only binds particular substrates.
These enzyme molecules bind the reactant molecules known as substrates at their active site forming an enzyme-substrate complex. This, in turn, brings up the reactants altogether and then positions them appropriately so the reaction can take place.
After the reaction completion, the products are released from the enzyme’s active site, which frees up the enzyme so that it can catalyze the other reactions.
However, the activities of these enzymes depend on the ionic conditions, the pH of the surroundings, and the temperature. Several enzymes function best at acidic pH while, in contrast, other function best in neutral environments.
Visualize the in-depth understanding of the natural world!
Biology would sound more interesting when your curiosity levels are satisfied with better visuals & logical explanations.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?