Enrich your knowledge with our informative blogs
What is a dative covalent bond?
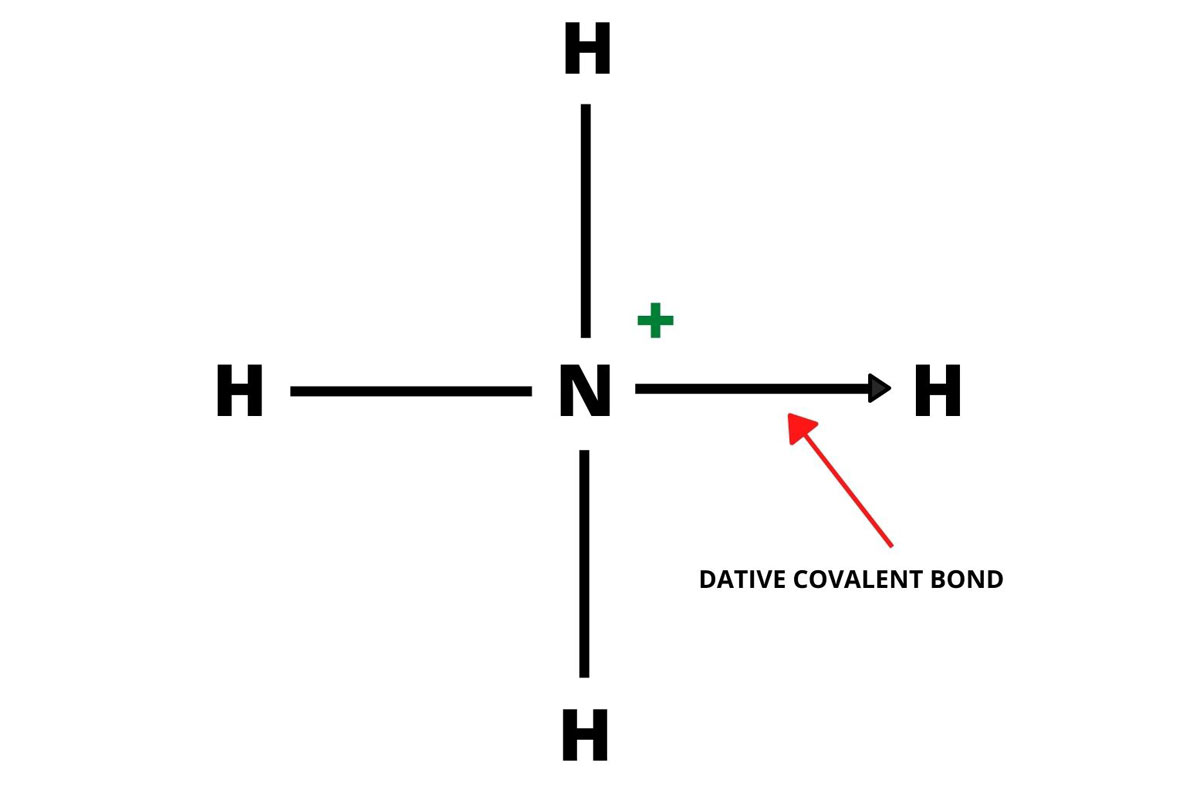
A dative covalent bond is a covalent bond that is formed by sharing electron pairs from a single atom. Both the shared electrons are donated by the same single atom. It is also called a co-ordinate covalent bond or dipolar bond. Electrons sharing takes place in a specific direction.
A dative covalent bond is a strong bond as the bonds are identical to any other interatomic bond.
These bonds are generally formed during reactions that involve two non-metals like a hydrogen atom or during the bond formation between ligands and metals ions.
Book Your 60-minutes Free Trial class NOW!
The bond is shown in the image is pointing in the direction where an atom is donating the lone pair to the atom that is receiving it.
Dative Bonds Possess Following Attributes: –
- The atom which shares an electron pair is called the donor (Lewis Base) and the atom which accepts these shared pair of electrons is called the receptor (Lewis Acid).
- The bond is illustrated by an arrow [→] from the donor atom towards the receptor atom.
- Atom gets stability after electron pair sharing.
- Dative covalent bonds have higher melting and boiling points than purely covalent bonds and lower melting and boiling points as compared to ionic compounds, hence, weaker than ionic bonding.
- They are rigid and directional and thus their coordinate compounds exhibit isomerism.
- These are bad conductors of electricity.
Book Your 60-minutes Free Trial class NOW!
Examples of Dative Covalent Bonds:-
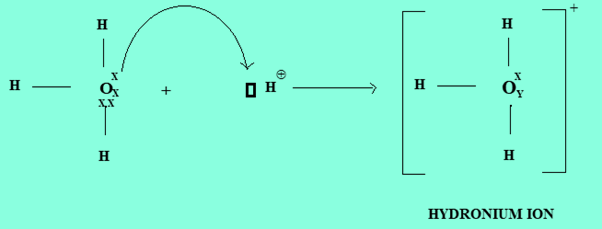
- Formation of Hydronium Ion
An Oxygen atom act as a donor atom and H+ act as a receptor. An oxygen atom in water donates its one pair of electrons to the vacant orbital of H+ ion forming a dative bond.
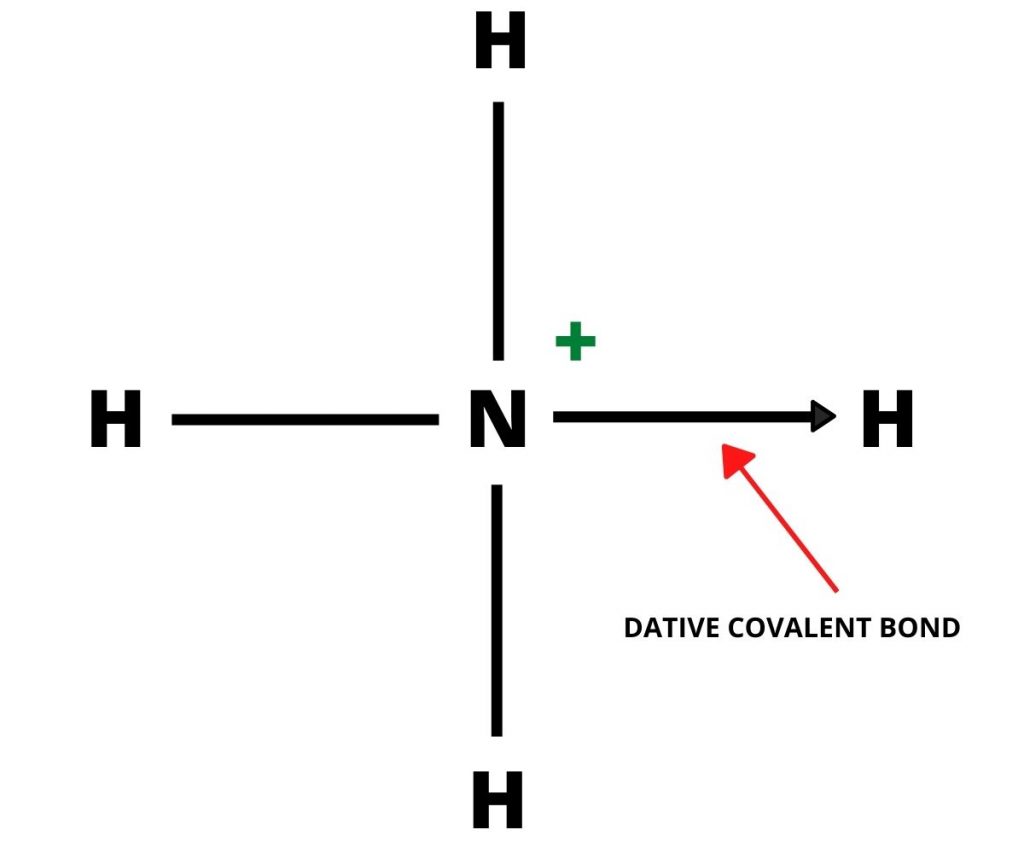
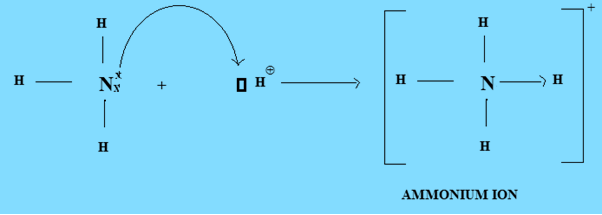
- Formation of Ammonium Ion
Nitrogen atom in Ammonia donates its electron pair to the vacant orbital of H+ ion. Nitrogen acts as a donor and H+ acts as an acceptor forming a dative covalent bond.

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?