Enrich your knowledge with our informative blogs
WHAT IS CONCENTRATION IN CHEMISTRY?
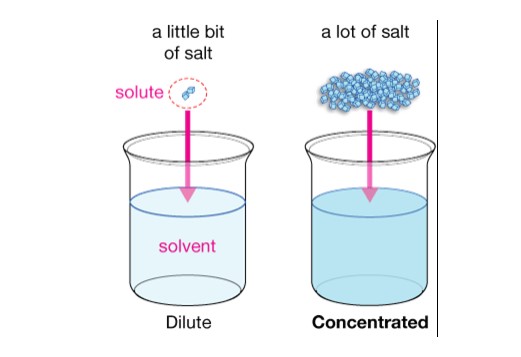
Concentration is the amount of solute added to a solution. Chemistry is not always related to chemist laboratories; we often deal with chemistry in our daily lives. Especially in the kitchen, reactions and process take place which directs to chemistry logics.
The process of adding solute to a given solution is known as Concentration, the more the amount of solute or substance, the greater the concentration. It is directly proportional to the strength of a solution.
Concentration has two types:
- Saturated Solution
- Unsaturated Solution
- A Solution to which we cannot add furthermore solute at a particular temperature is ‘Saturated Solution.’
- A solution to which increased solute can be added and dissolved at the same temperature is known as an ‘Unsaturated Solution.’
Mathematically,
Concentration =
To understand the concept of Concentration in chemistry, let us take an example of lemon juice. For this, you need to perform an experiment in which you need the following items.
- Salt
- Water
- A Bowl or a glass
Take a half glass of water and dissolve two spoons of common salt in it. Here, water acts as a solvent and we take Salt as a solute. On drinking the water, you will see that the taste is stronger because we added more amount of lemon drops into only a half glass of water.
Simply, we can say the solution is More Concentrated if more solute is added, whereas on mixing a little amount the solution we get will be ‘Dilute.’

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?