Enrich your knowledge with our informative blogs
What Is Displacement In Chemistry?
A displacement reaction is a reaction where a part of the reactant replaces another reactant. It is also known as the metathesis or replacement reaction.
The displacement reactions involve metal and a compound of a different metal. Talking about it, in a displacement reaction, “A more reactive metal will displace a less reactive metal from its compounds.”
A displacement reaction is easily seen when the salt of less reactive metal is in the solution. During the reaction, the more reactive metal gradually disappears as it forms a solution, whereas the less reactive metal coats the surface of the more reactive metal.
Book Your 60-minutes Free Trial class NOW!
There are two types of displacement reactions.
1. Single Displacement Reaction
A single displacement reaction is one where one reactant replaces the part of another reactant. In short, it follows the below-mentioned mechanism:
AB + C AC + B
Example:
Fe + CuSO4 FeSO4 + Cu
2. Double Displacement Reaction
Double Displacement reactions are the ones where the anions and cations in the reactants switch their partners to produce the products. It follows the below-mentioned mechanism:
AB + CD AD + CB
Example:
AgNO3 + NaCl AgCl + NaNO3
Book Your 60-minutes Free Trial class NOW!
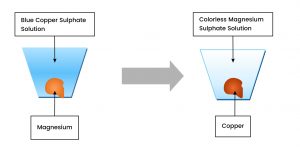
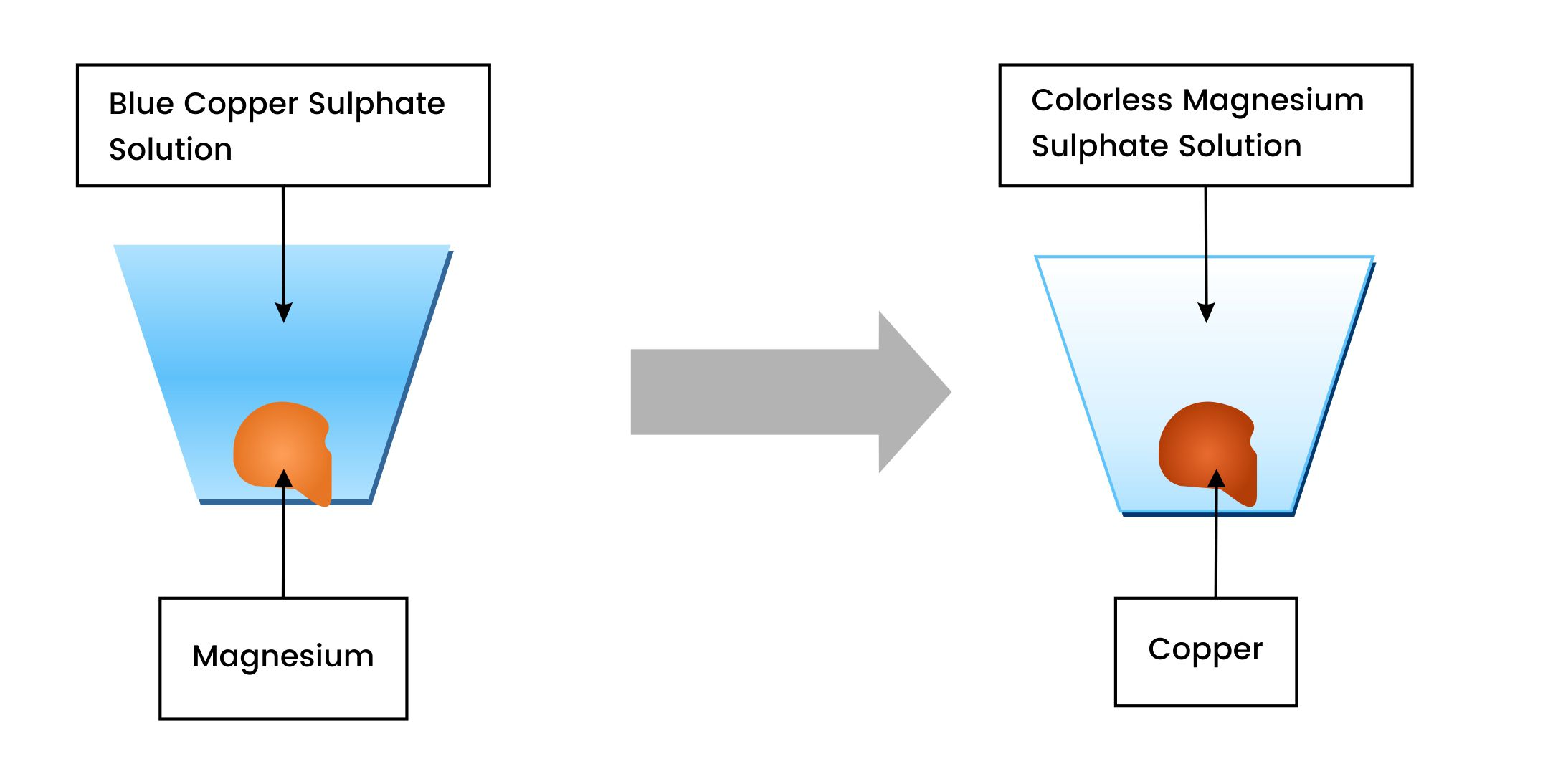
For instance, let us consider the two metals, magnesium and copper, as we know that magnesium is more reactive than copper.
When a piece of magnesium is dipped into a copper sulfate solution, the blue colour lightens due to the formation of colorless magnesium sulfate solution, and the brown copper coats the magnesium surface.
The equation for this can be as follow:
Magnesium + Copper Sulfate Solution Magnesium Sulfate + Copper
Mg + CuSO4 MgSO4 + Cu
Did you find it relevant? Don’t forget to check on other essential topics of the subjects of your interest.
Read More – Chemistry Questions

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?