Enrich your knowledge with our informative blogs
WHAT IS DISSOLUTION IN CHEMISTRY?

In Chemistry, we deal with solutes and solvents. Dissolution is the process of mixing solute and solvent to make a new formation. The solute can be any matter like solid, liquid or gas. The mixing of the solute and solvent components forms a solution, the process of dissolving the particles and forming a new product is called “dissolution”
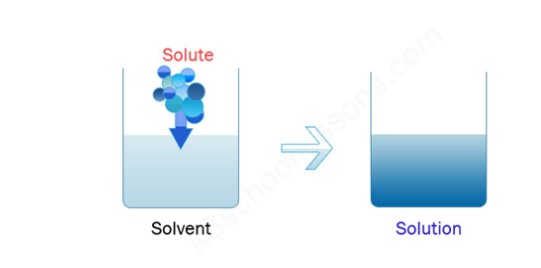
Solute + Solvent = Solution
A solution is the homogeneous mixing of two or more bound components.
The above figure shows an example of ‘Dissolution’
- A solvent is the main component present in a solution that acts as a dissolving agent.
- A solute is a substance that is dissolved in a solvent for the formation of a “solution”.
- Polar solutes dissolve in a polar solvent and non-polar solutes can be readily dissolved in non-polar solvents.
In Dissolution, the resulting product is formed by attractive forces between the atoms or molecules involved in a substance.
Book Your 60-minutes Free Trial class NOW!

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?