Enrich your knowledge with our informative blogs
What is k in chemistry?
“K” refers to the equilibrium constant of a chemical reaction. It provides insight into the relationship between the reactants and products when a chemical reaction reaches equilibrium.
For instance,
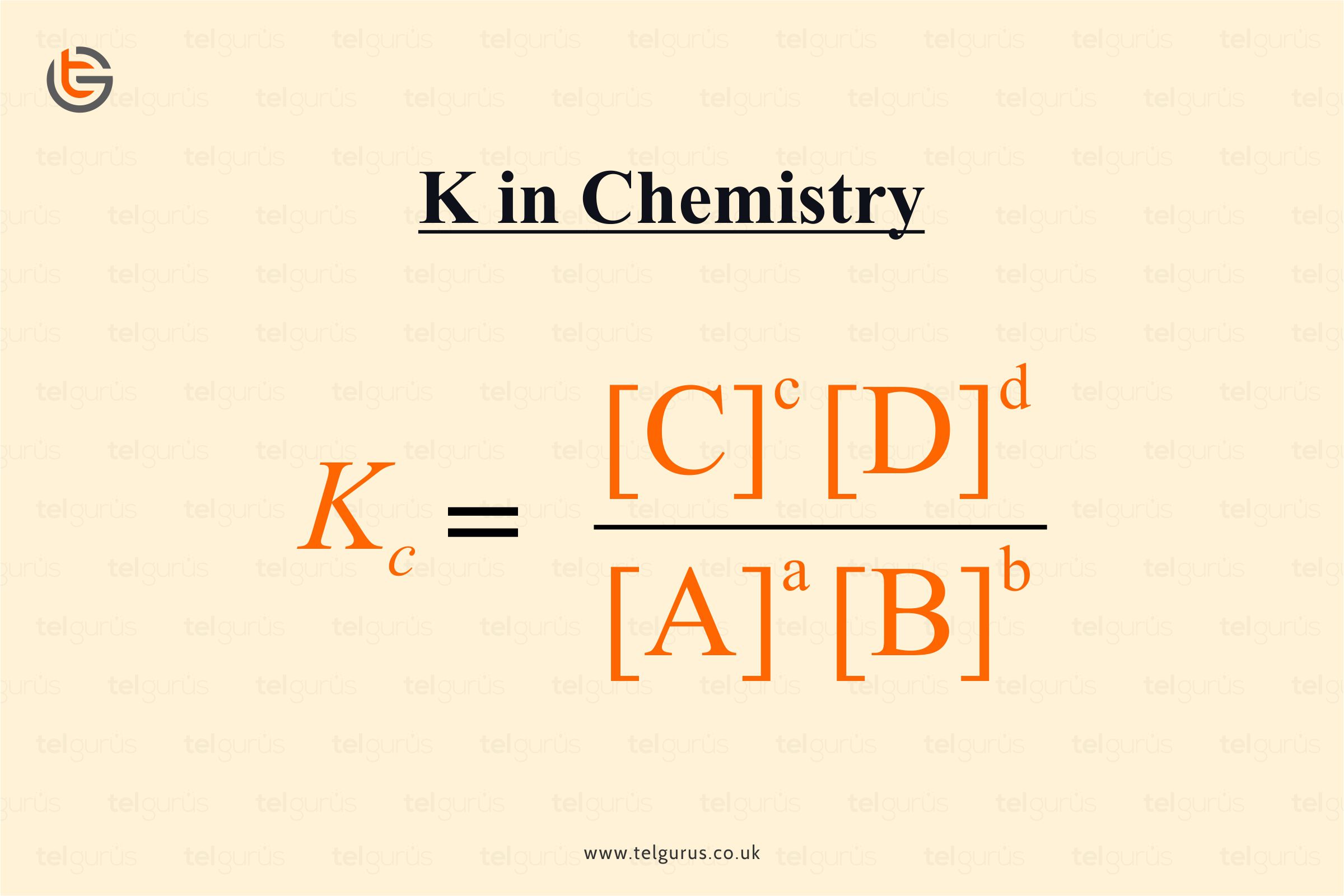
The concentration’s equilibrium constant denoted by Kc of a chemical reaction can be defined as the ratio of product’s concentration to reactant’s concentration. Each one raised their respective stoichiometric coefficients.
However, it is essential to be noted that the equilibrium constants are of several types that endow with relationships between reactants and products of equilibrium reactions in terms of different units.
At equilibrium, Forward reaction’s rate = Backward reaction’s rate
i.e. rf= rb
Given a reaction,
aA + bB ⇌ cC + dD
For the above reaction, the equilibrium constant is defined as:
Where Kc, indicates the equilibrium constant that is measured in moles per litre.
The unit of equilibrium constant = [Mole L-1] △n
Where △n= Sum of product’s stoichiometric coefficients – Sum of reactant’s stoichiometric coefficients.
Read More – Chemistry Questions
View More – Useful links for Your Child’s Development

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?