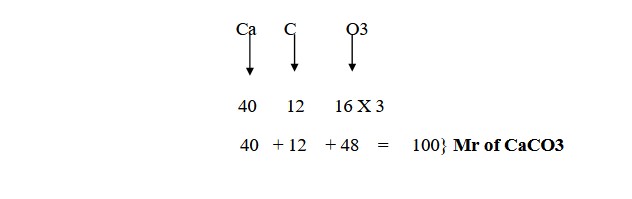Enrich your knowledge with our informative blogs
What is “Mr” and “Ka” in Chemistry?

What is “Mr” in Chemistry?
“Mr” stands for relative formula mass that can be used in the conservation of mass calculations. Calculations can be done to figure out the uncertainties in measurements and concentrations of a solution.
Calculation of relative formula mass
The relative formula mass of a substance is made up of molecules. A relative formula mass Mr of a compound can be calculated as the sum of relative atomic masses of all the atoms in the formula’s number. Ar is the relative molecular mass in the periodic table.
Find the Mr of CO.
Ar of O (Oxygen) is 16 and Ar of C (Carbon) is 12
Mr of CO = 16 + 12 = 28.
Let us take another example of this.
For instance, you need to calculate the relative formula mass Mr of CaCO3, where it is given that the relative atomic mass of Calcium is 40; Oxygen is 16 and Carbon is 12.
Now let us see how we can calculate the Mr
Book Your 60-minutes Free Trial class NOW!
What is “Ka” in Chemistry?
The “Ka” stands for the acid dissociation constant. It is the equilibrium constant of the dissociation reaction of an acid. Ka is commonly expressed in the units of mol/L.
There are tables of the acid dissociation constant. In the case of an aqueous solution, the general form of equilibrium reaction is:
HA + H2O ⇆ A- + H3O+
Here HA is an acid that dissociates in the conjugate base of A–
A hydrogen ion combines water to form the hydronium ion H3O+
When the concentrations of HA, H3O+, and A– no longer changed over time, then the reaction is said to be at equilibrium, and the dissociation constant for it may be calculated as:
Ka = [A- ] [H3O+ ] / [HA] [ H2O]
You must be wondering why we have used the brackets here. The square brackets indicate the concentration.
Unless an acid is tremendously concentrated, the equation is simplified by holding the water concentration as constant. Let us see how we can represent it.
HA ⇆ A- + H+
Ka = [A- ] [H+ ] / [HA]
The acid dissociation constant is also referred to as acid ionization constant or acidity constant.

Discover the exact logic behind the reactions!
Get a deeper understanding of every possible interaction between atoms, molecules and elements in an easy and fun-loving way.
Categories
Recent Posts
- List of the qualities you should look for in your tutors?
- What is the most useful formulas in math?
- Describe the process of eating to defecation of food?
- Difference between the natural and artificial active response by the immunology system.
- Explain the different circle theorems
- How are nerve cells adapted to their function?